Development of a human immunodeficiency virus vector-based, single-cycle assay for evaluation of anti-integrase compounds
- PMID: 17005823
- PMCID: PMC1610086
- DOI: 10.1128/AAC.00517-06
Development of a human immunodeficiency virus vector-based, single-cycle assay for evaluation of anti-integrase compounds
Abstract
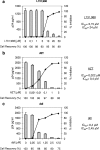
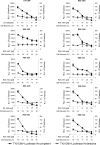
Therapeutic strategies aimed at inhibiting human immunodeficiency virus type 1 (HIV-1) replication employ a combination of drugs targeted to two viral enzymes (reverse transcriptase and protease) and to the viral entry/fusion step. However, the high propensity of HIV-1 to develop resistance makes the development of novel compounds targeting different steps of the HIV-1 life cycle essential. Among these, integrase (IN) inhibitors have successfully passed the early phases of clinical development. By preventing integration, IN inhibitors preclude viral replication while allowing production of extrachromosomal forms of viral DNA (E-DNA). Here, we describe an improved and standardized assay aimed at evaluating IN inhibitors by taking advantage of the transcriptional activity of E-DNA produced by HIV-derived vectors in the absence of replication-competent virus. In this context, the use of the firefly luciferase gene as a reporter gene provides a rapid and quantitative measure of viral-vector infectivity, thus making it a safe and cost-effective assay for evaluating novel IN inhibitors.
Figures



Similar articles
-
The naphthyridinone GSK364735 is a novel, potent human immunodeficiency virus type 1 integrase inhibitor and antiretroviral.Antimicrob Agents Chemother. 2008 Mar;52(3):901-8. doi: 10.1128/AAC.01218-07. Epub 2007 Dec 26. Antimicrob Agents Chemother. 2008. PMID: 18160521 Free PMC article.
-
Evaluation of HIV-1 integrase inhibitors on human primary macrophages using a luciferase-based single-cycle phenotypic assay.J Virol Methods. 2010 Sep;168(1-2):272-6. doi: 10.1016/j.jviromet.2010.06.004. Epub 2010 Jun 15. J Virol Methods. 2010. PMID: 20558207
-
[Universal modular system for in vitro screening of potential inhibitors of HIV-1 replication].Mol Biol (Mosk). 2014 Mar-Apr;48(2):344-8. Mol Biol (Mosk). 2014. PMID: 25850304 Russian.
-
HIV type 1 integrase inhibitors: from basic research to clinical implications.AIDS Rev. 2008 Jul-Sep;10(3):172-89. AIDS Rev. 2008. PMID: 18820719 Review.
-
Recent progress in the development of inhibitors of human immunodeficiency virus (HIV) integrase for the management of HIV infection.Acta Virol. 2008;52(4):197-207. Acta Virol. 2008. PMID: 19143475 Review.
Cited by
-
Nonintegrating Lentiviral Vector-Based Vaccine Efficiently Induces Functional and Persistent CD8+ T Cell Responses in Mice.J Biomed Biotechnol. 2010;2010:534501. doi: 10.1155/2010/534501. Epub 2010 May 19. J Biomed Biotechnol. 2010. PMID: 20508727 Free PMC article.
-
Development and use of SIV-based Integrase defective lentiviral vector for immunization.Vaccine. 2009 Jul 23;27(34):4622-9. doi: 10.1016/j.vaccine.2009.05.070. Epub 2009 Jun 11. Vaccine. 2009. PMID: 19523909 Free PMC article.
-
Gag determinants of fitness and drug susceptibility in protease inhibitor-resistant human immunodeficiency virus type 1.J Virol. 2009 Sep;83(18):9094-101. doi: 10.1128/JVI.02356-08. Epub 2009 Jul 8. J Virol. 2009. PMID: 19587031 Free PMC article.
-
Human immunodeficiency virus type 1 (HIV-1) integration: a potential target for microbicides to prevent cell-free or cell-associated HIV-1 infection.Antimicrob Agents Chemother. 2008 Jul;52(7):2544-54. doi: 10.1128/AAC.01627-07. Epub 2008 May 12. Antimicrob Agents Chemother. 2008. PMID: 18474579 Free PMC article.
-
Effects of raltegravir on 2-long terminal repeat circle junctions in HIV type 1 viremic and aviremic patients.AIDS Res Hum Retroviruses. 2013 Oct;29(10):1365-9. doi: 10.1089/AID.2013.0047. Epub 2013 Jul 24. AIDS Res Hum Retroviruses. 2013. PMID: 23802629 Free PMC article.
References
-
- Ansari-Lari, M. A., L. Donehower, and R. A. Gibbs. 1995. Analysis of human immunodeficiency virus type 1 integrase mutants. Virology 211:332-335. - PubMed
-
- Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631-634. - PubMed
-
- Cara, A., F. Guarnaccia, M. S. Reitz, Jr., R. C. Gallo, and F. Lori. 1995. Self-limiting, cell type-dependent replication of an integrase-defective human immunodeficiency virus type 1 in human primary macrophages but not T lymphocytes. Virology 208:242-248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

