Na+, Cl-, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: the proton inactivation hypothesis of hCHT in synaptic vesicles
- PMID: 17005849
- PMCID: PMC6674471
- DOI: 10.1523/JNEUROSCI.1862-06.2006
Na+, Cl-, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: the proton inactivation hypothesis of hCHT in synaptic vesicles
Abstract
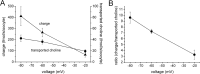
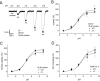
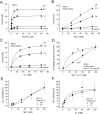
The recent cloning of the human choline transporter (hCHT) has allowed its expression in Xenopus laevis oocytes and the simultaneous measurement of choline transport and choline-induced current under voltage clamp. hCHT currents and choline transport are evident in cRNA-injected oocytes and significantly enhanced by the hCHT trafficking mutant L530A/V531A. The charge/choline ratio of hCHT varies from 10e/choline at -80 mV to 3e/choline at -20 mV, in contrast with the reported fixed stoichiometry of the Na+-coupled glucose transporter in the same gene family. Ion substitution shows that the choline uptake and choline-induced current are Na+ and Cl- dependent; however, the reversal potential of the induced current suggests a Na+-selective mechanism, consigning Cl- to a regulatory role rather than a coupled, cotransported-ion role. The hCHT-specific inhibitor hemicholinium-3 (HC-3) blocks choline uptake and choline-induced current; in addition, HC-3 alone reveals a constitutive, depolarizing leak current through hCHT. We show that external protons reduce hCHT current, transport, and binding with a similar pKa of 7.4, suggesting proton titration of residue(s) that support choline binding and transport. Given the localization of the choline transporter to synaptic vesicles, we propose that proton inactivation of hCHT prevents acetylcholine and proton leakage from the acidic interior of cholinergic synaptic vesicles. This mechanism would allow cholinergic, activity-triggered delivery of silent choline transporters to the plasma membrane, in which normal pH would reactivate the transporters for choline uptake and subsequent acetylcholine synthesis.
Figures





Similar articles
-
Molecular cloning of a human, hemicholinium-3-sensitive choline transporter.Biochem Biophys Res Commun. 2000 Oct 5;276(3):862-7. doi: 10.1006/bbrc.2000.3561. Biochem Biophys Res Commun. 2000. PMID: 11027560
-
GAT1 (GABA:Na+:Cl-) cotransport function. Steady state studies in giant Xenopus oocyte membrane patches.J Gen Physiol. 1999 Sep;114(3):429-44. doi: 10.1085/jgp.114.3.429. J Gen Physiol. 1999. PMID: 10469733 Free PMC article.
-
The hemicholinium-3 sensitive high affinity choline transporter is internalized by clathrin-mediated endocytosis and is present in endosomes and synaptic vesicles.J Neurochem. 2003 Oct;87(1):136-46. doi: 10.1046/j.1471-4159.2003.01974.x. J Neurochem. 2003. PMID: 12969261
-
The choline transporter resurfaces: new roles for synaptic vesicles?Mol Interv. 2004 Feb;4(1):22-37. doi: 10.1124/mi.4.1.22. Mol Interv. 2004. PMID: 14993474 Review.
-
Modulation of sodium-coupled choline transporter CHT function in health and disease.Neurochem Int. 2020 Nov;140:104810. doi: 10.1016/j.neuint.2020.104810. Epub 2020 Aug 5. Neurochem Int. 2020. PMID: 32768485 Review.
Cited by
-
Structural basis of lipid head group entry to the Kennedy pathway by FLVCR1.Nature. 2024 May;629(8012):710-716. doi: 10.1038/s41586-024-07374-4. Epub 2024 May 1. Nature. 2024. PMID: 38693265 Free PMC article.
-
Identification and characterization of ML352: a novel, noncompetitive inhibitor of the presynaptic choline transporter.ACS Chem Neurosci. 2015 Mar 18;6(3):417-27. doi: 10.1021/cn5001809. Epub 2015 Feb 2. ACS Chem Neurosci. 2015. PMID: 25560927 Free PMC article.
-
Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of "bath salts," produce opposite effects at the human dopamine transporter.Psychopharmacology (Berl). 2013 Jun;227(3):493-9. doi: 10.1007/s00213-013-2967-2. Epub 2013 Jan 31. Psychopharmacology (Berl). 2013. PMID: 23371489 Free PMC article.
-
Substrate-induced internalization of the high-affinity choline transporter.J Neurosci. 2011 Oct 19;31(42):14989-97. doi: 10.1523/JNEUROSCI.2983-11.2011. J Neurosci. 2011. PMID: 22016532 Free PMC article.
-
Distinct Cation Gradients Power Cholesterol Transport at Different Key Points in the Hedgehog Signaling Pathway.Dev Cell. 2020 Nov 9;55(3):314-327.e7. doi: 10.1016/j.devcel.2020.08.002. Epub 2020 Aug 28. Dev Cell. 2020. PMID: 32860743 Free PMC article.
References
-
- Apparsundaram S, Ferguson SM, George AL, Jr, Blakely RD. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem Biophys Res Commun. 2000;276:862–867. - PubMed
-
- Apparsundaram S, Ferguson SM, Blakely RD. Molecular cloning and characterization of a murine hemicholinium-3-sensitive choline transporter. Biochem Soc Trans. 2001;29:711–716. - PubMed
-
- Aubrey KR, Mitrovic AD, Vandenberg RJ. Molecular basis for proton regulation of glycine transport by glycine transporter subtype 1b. Mol Pharmacol. 2000;58:129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
