Beta-arrestin2-mediated internalization of mammalian odorant receptors
- PMID: 17005854
- PMCID: PMC6674477
- DOI: 10.1523/JNEUROSCI.2897-06.2006
Beta-arrestin2-mediated internalization of mammalian odorant receptors
Abstract
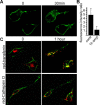
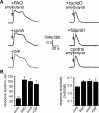
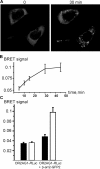
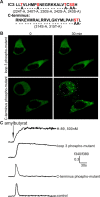
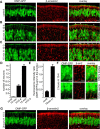
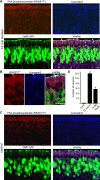
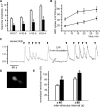
Odorant receptors comprise the biggest subfamily of G-protein-coupled receptors. Although the endocytic mechanisms of other G-protein-coupled receptors have been characterized extensively, almost nothing is known about the intracellular trafficking of odorant receptors. The present study describes the endocytic pathway of mammalian odorant receptors, which bind beta-arrestin2 with high affinity and are internalized via a clathrin-dependent mechanism. After prolonged odorant exposure, receptors are not targeted to lysosomal degradation but accumulate in recycling endosomes. Odorant-induced odorant receptor desensitization is promoted by cAMP-dependent protein kinase A phosphorylation and is dependent on serine and threonine residues within the third intracellular loop of the receptor. Moreover, beta-arrestin2 is redistributed into the dendritic knobs of mouse olfactory receptor neurons after treatment with a complex odorant mixture. Prolonged odorant exposure resulted in accumulation of beta-arrestin2 in intracellular vesicles. Adaptation of olfactory receptor neurons to odorants can be abolished by the inhibition of clathrin-mediated endocytosis, showing the physiological relevance of the here described mechanism of odorant receptor desensitization. A better understanding of odorant receptor trafficking and additional insight into the molecular determinants underlying the interactions of odorant receptors with beta-arrestin2 and other trafficking proteins will therefore be important to fully understand the mechanisms of adaptation and sensitization in the olfactory epithelium.
Figures
Similar articles
-
Properties of secretin receptor internalization differ from those of the beta(2)-adrenergic receptor.J Biol Chem. 1999 Oct 29;274(44):31515-23. doi: 10.1074/jbc.274.44.31515. J Biol Chem. 1999. PMID: 10531354
-
Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors.Mol Endocrinol. 2000 Dec;14(12):2040-53. doi: 10.1210/mend.14.12.0565. Mol Endocrinol. 2000. PMID: 11117533
-
Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases.J Neurochem. 2006 Feb;96(4):934-49. doi: 10.1111/j.1471-4159.2005.03603.x. Epub 2006 Jan 12. J Neurochem. 2006. PMID: 16412099
-
Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling.Pharmacol Rev. 2001 Mar;53(1):1-24. Pharmacol Rev. 2001. PMID: 11171937 Review.
-
Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins.Prog Neurobiol. 2002 Feb;66(2):61-79. doi: 10.1016/s0301-0082(01)00023-5. Prog Neurobiol. 2002. PMID: 11900882 Review.
Cited by
-
Human olfactory receptor responses to odorants.Sci Data. 2015 Feb 3;2:150002. doi: 10.1038/sdata.2015.2. eCollection 2015. Sci Data. 2015. PMID: 25977809 Free PMC article.
-
Profiling of olfactory receptor gene expression in whole human olfactory mucosa.PLoS One. 2014 May 6;9(5):e96333. doi: 10.1371/journal.pone.0096333. eCollection 2014. PLoS One. 2014. PMID: 24800820 Free PMC article.
-
Hedgehog signaling regulates ciliary localization of mouse odorant receptors.Proc Natl Acad Sci U S A. 2017 Oct 31;114(44):E9386-E9394. doi: 10.1073/pnas.1708321114. Epub 2017 Oct 16. Proc Natl Acad Sci U S A. 2017. PMID: 29078327 Free PMC article.
-
Human Olfactory Receptor Sensor for Odor Reconstitution.Sensors (Basel). 2023 Jul 5;23(13):6164. doi: 10.3390/s23136164. Sensors (Basel). 2023. PMID: 37448013 Free PMC article. Review.
-
The membrane proteome of sensory cilia to the depth of olfactory receptors.Mol Cell Proteomics. 2014 Jul;13(7):1828-43. doi: 10.1074/mcp.M113.035378. Epub 2014 Apr 18. Mol Cell Proteomics. 2014. PMID: 24748648 Free PMC article.
References
-
- Arshavsky VY. Protein translocation in photoreceptor light adaptation: a common theme in vertebrate and invertebrate vision. Sci STKE. 2003;2003:E43. - PubMed
-
- Bannister LH, Dodson HC. Endocytic pathways in the olfactory and vomeronasal epithelia of the mouse: ultrastructure and uptake of tracers. Microsc Res Tech. 1992;23:128–141. - PubMed
-
- Barnea G, O’Donnell S, Mancia F, Sun X, Nemes A, Mendelsohn M, Axel R. Odorant receptors on axon termini in the brain. Science. 2004;304:1468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
