Global transcriptome analysis of genetically identified neurons in the adult cortex
- PMID: 17005859
- PMCID: PMC6674475
- DOI: 10.1523/JNEUROSCI.0468-06.2006
Global transcriptome analysis of genetically identified neurons in the adult cortex
Abstract
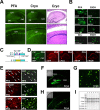
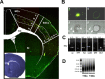
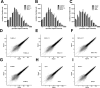
The enormous cellular complexity of the brain is a major obstacle for gene expression profiling of neurological disease models, because physiologically relevant changes of transcription in a specific neuronal subset are likely to be lost in the presence of other neurons and glia. We solved this problem in transgenic mice by labeling genetically defined cells with a nuclear variant of GFP. When combined with laser-directed microdissection, intact RNA from unfixed, freeze-dried sections can be isolated, which is a prerequisite for high-quality global transcriptome analysis. Here, we compared gene expression profiles between pyramidal motor neurons and pyramidal somatosensory neurons captured from layer V of the adult neocortex. One striking feature of motor neurons is the elevated expression of ribosomal genes and genes involved in ATP synthesis. This suggests a molecular adaptation of the upper motor neurons to longer axonal projections and higher electrical activity. These molecular signatures were not detected when cortical layers and microareas were analyzed in toto. Additionally, we used microarrays to determine the global mRNA expression profiles of microdissected Purkinje cells and cellularly complex cerebellar cortex microregions. In summary, our analysis shows that cellularly complex targets lead to averaged gene expression profiles that lack substantial amounts of cell type-specific information. Thus, cell type-restricted sampling strategies are mandatory in the CNS. The combined use of a genetic label with laser-microdissection offers an unbiased approach to map patterns of gene expression onto practically any cell type of the brain.
Figures


Similar articles
-
Microarray analysis of fluoro-gold labeled rat dopamine neurons harvested by laser capture microdissection.J Neurosci Methods. 2005 Apr 30;143(2):95-106. doi: 10.1016/j.jneumeth.2004.09.023. Epub 2004 Nov 28. J Neurosci Methods. 2005. PMID: 15814141
-
Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder.Mol Psychiatry. 2015 Nov;20(11):1397-405. doi: 10.1038/mp.2014.171. Epub 2015 Jan 6. Mol Psychiatry. 2015. PMID: 25560755 Free PMC article.
-
Laser microdissection of immunolabeled astrocytes allows quantification of astrocytic gene expression.J Neurosci Methods. 2004 Sep 30;138(1-2):141-8. doi: 10.1016/j.jneumeth.2004.03.022. J Neurosci Methods. 2004. PMID: 15325122
-
Characterization of mRNA expression in single neurons.Methods Mol Biol. 2007;399:133-52. doi: 10.1007/978-1-59745-504-6_10. Methods Mol Biol. 2007. PMID: 18309930 Review.
-
Gene expression profiling in the post-mortem human brain--no cause for dismay.J Chem Neuroanat. 2001 Jul;22(1-2):79-94. doi: 10.1016/s0891-0618(01)00099-0. J Chem Neuroanat. 2001. PMID: 11470556 Review.
Cited by
-
Combinatorial expression rules of ion channel genes in juvenile rat (Rattus norvegicus) neocortical neurons.PLoS One. 2012;7(4):e34786. doi: 10.1371/journal.pone.0034786. Epub 2012 Apr 11. PLoS One. 2012. PMID: 22509357 Free PMC article.
-
The molecular mechanisms of cell death in the course of transient ischemia are differentiated in evolutionary distinguished brain structures.Metab Brain Dis. 2009 Sep;24(3):507-23. doi: 10.1007/s11011-009-9149-2. Epub 2009 Aug 20. Metab Brain Dis. 2009. PMID: 19693659
-
P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes.J Neurosci. 2008 May 21;28(21):5473-80. doi: 10.1523/JNEUROSCI.1149-08.2008. J Neurosci. 2008. PMID: 18495881 Free PMC article.
-
Role of GABAA-Mediated Inhibition and Functional Assortment of Synapses onto Individual Layer 4 Neurons in Regulating Plasticity Expression in Visual Cortex.PLoS One. 2016 Feb 3;11(2):e0147642. doi: 10.1371/journal.pone.0147642. eCollection 2016. PLoS One. 2016. PMID: 26841221 Free PMC article.
-
Age-dependent modulation of cortical transcriptomes in spinal cord injury and repair.PLoS One. 2012;7(12):e49812. doi: 10.1371/journal.pone.0049812. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236355 Free PMC article.
References
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. - PubMed
-
- Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB. Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem Res. 2001;26:739–764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
