Targeted deletion of a single Sca8 ataxia locus allele in mice causes abnormal gait, progressive loss of motor coordination, and Purkinje cell dendritic deficits
- PMID: 17005861
- PMCID: PMC6674467
- DOI: 10.1523/JNEUROSCI.2595-06.2006
Targeted deletion of a single Sca8 ataxia locus allele in mice causes abnormal gait, progressive loss of motor coordination, and Purkinje cell dendritic deficits
Abstract
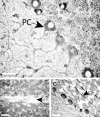
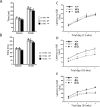
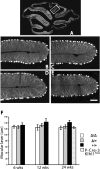
Spinocerebellar ataxia type 8 (SCA8) patients typically have a slowly progressive, adult-onset ataxia. SCA8 is dominantly inherited and is caused by large CTG repeat expansions in the untranslated antisense RNA of the Kelch-like 1 gene (KLHL1), but the molecular mechanism through which this expansion leads to disease is still unknown. To more fully characterize the underlying molecular mechanisms involved in SCA8, we developed a mouse model in which Klhl1 is deleted in either all tissues or is deleted specifically in Purkinje cells only. We found that mice that are either homozygous or heterozygous for the Klhl1 deletion have significant gait abnormalities at an early age and develop a significant loss of motor coordination by 24 weeks of age. This loss progresses more rapidly in homozygous knock-outs. Mice with Klhl1 specifically deleted in only Purkinje cells had a loss of motor coordination that was almost identical to the total-tissue deletion mice. Finally, we found significant Purkinje cell dendritic deficits, as measured by the thickness of the molecular layer, in all mice in which Klhl1 was deleted (both total and Purkinje cell-specific deletions) and an intermediate reduction in molecular layer thickness in mice with reduced levels of Klhl1 expression (heterozygous deletions). The results from this mouse model show that even a partial loss of Klhl1 function leads to degeneration of Purkinje cell function and indicates that loss of KLHL1 activity is likely to play a significant part in the underlying pathophysiology of SCA8.
Figures


Similar articles
-
The Kelch-like protein 1 modulates P/Q-type calcium current density.Neuroscience. 2007 Mar 30;145(3):841-50. doi: 10.1016/j.neuroscience.2006.12.046. Epub 2007 Feb 6. Neuroscience. 2007. PMID: 17289272
-
SCA8 mRNA expression suggests an antisense regulation of KLHL1 and correlates to SCA8 pathology.Brain Res. 2008 Oct 3;1233:176-84. doi: 10.1016/j.brainres.2008.07.096. Epub 2008 Aug 3. Brain Res. 2008. PMID: 18708037
-
The KLHL1-antisense transcript ( KLHL1AS) is evolutionarily conserved.Mamm Genome. 2002 Mar;13(3):134-41. doi: 10.1007/s00335-001-2105-2. Mamm Genome. 2002. PMID: 11919683
-
Calcium Signaling, PKC Gamma, IP3R1 and CAR8 Link Spinocerebellar Ataxias and Purkinje Cell Dendritic Development.Curr Neuropharmacol. 2018 Jan 30;16(2):151-159. doi: 10.2174/1570159X15666170529104000. Curr Neuropharmacol. 2018. PMID: 28554312 Free PMC article. Review.
-
Molecular genetics of spinocerebellar ataxia type 8 (SCA8).RNA Biol. 2005 Apr;2(2):49-52. doi: 10.4161/rna.2.2.1682. Epub 2005 Apr 25. RNA Biol. 2005. PMID: 17132942 Review.
Cited by
-
Animal models of human cerebellar ataxias: a cornerstone for the therapies of the twenty-first century.Cerebellum. 2009 Sep;8(3):137-54. doi: 10.1007/s12311-009-0127-3. Cerebellum. 2009. PMID: 19669387
-
Internal ribosome entry segment activity of ATXN8 opposite strand RNA.PLoS One. 2013 Sep 11;8(9):e73885. doi: 10.1371/journal.pone.0073885. eCollection 2013. PLoS One. 2013. PMID: 24040107 Free PMC article.
-
Identification of Dlk1, Ptpru and Klhl1 as novel Nurr1 target genes in meso-diencephalic dopamine neurons.Development. 2009 Jul;136(14):2363-73. doi: 10.1242/dev.037556. Epub 2009 Jun 10. Development. 2009. PMID: 19515692 Free PMC article.
-
Persistent motor dysfunction despite homeostatic rescue of cerebellar morphogenesis in the Car8 waddles mutant mouse.Neural Dev. 2019 Mar 12;14(1):6. doi: 10.1186/s13064-019-0130-4. Neural Dev. 2019. PMID: 30867000 Free PMC article.
-
Kelch proteins: emerging roles in skeletal muscle development and diseases.Skelet Muscle. 2014 Jun 1;4:11. doi: 10.1186/2044-5040-4-11. eCollection 2014. Skelet Muscle. 2014. PMID: 24959344 Free PMC article. Review.
References
-
- Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. 1995;6:1193–1198. - PubMed
-
- Bardwell VJ, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994;8:1664–1677. - PubMed
-
- Benzow KA, Koob MD. The KLHL1-antisense transcript (KLHL1AS) is evolutionarily conserved. Mamm Genome. 2002;13:134–141. - PubMed
-
- Bomont P, Cavalier L, Blondeau F, Ben Hamida C, Belal S, Tazir M, Demir E, Topaloglu H, Korinthenberg R, Tuysuz B, Landrieu P, Hentati F, Koenig M. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet. 2000;26:370–374. - PubMed
-
- Chen Y, Li M. Interactions between CAP70 and actinfilin are important for integrity of actin cytoskeleton structures in neurons. Neuropharmacology. 2005;49:1026–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
