Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS
- PMID: 17005864
- PMCID: PMC6674481
- DOI: 10.1523/JNEUROSCI.2580-06.2006
Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS
Abstract
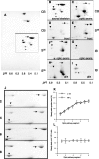
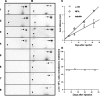
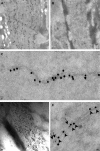
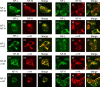
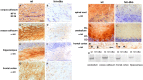
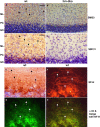
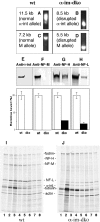
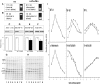
Alpha-internexin, a neuronal intermediate filament protein implicated in neurodegenerative disease, coexists with the neurofilament (NF) triplet proteins (NF-L, NF-M, and NF-H) but has an unknown function. The earlier peak expression of alpha-internexin than the triplet during brain development and its ability to form homopolymers, unlike the triplet, which are obligate heteropolymers, have supported a widely held view that alpha-internexin and neurofilament triplet form separate filament systems. Here, we demonstrate, however, that despite a postnatal decline in expression, alpha-internexin is as abundant as the triplet in the adult CNS and exists in a relatively fixed stoichiometry with these subunits. Alpha-internexin exhibits transport and turnover rates identical to those of triplet proteins in optic axons and colocalizes with NF-M on single neurofilaments by immunogold electron microscopy. Alpha-internexin also coassembles with all three neurofilament proteins into a single network of filaments in quadruple-transfected SW13vim(-) cells. Genetically deleting NF-M alone or together with NF-H in mice dramatically reduces alpha-internexin transport and content in axons throughout the CNS. Moreover, deleting alpha-internexin potentiates the effects of NF-M deletion on NF-H and NF-L transport. Finally, overexpressing a NF-H-LacZ fusion protein in mice induces alpha-internexin and neurofilament triplet to aggregate in neuronal perikarya and greatly reduces their transport and content selectively in axons. Our data show that alpha-internexin and the neurofilament proteins are functionally interdependent. The results strongly support the view that alpha-internexin is a fourth subunit of neurofilaments in the adult CNS, providing a basis for its close relationship with neurofilaments in CNS diseases associated with neurofilament accumulation.
Figures


Similar articles
-
Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons.J Neurosci. 2012 Jun 20;32(25):8501-8. doi: 10.1523/JNEUROSCI.1081-12.2012. J Neurosci. 2012. PMID: 22723690 Free PMC article.
-
Neurofilament transport in vivo minimally requires hetero-oligomer formation.J Neurosci. 2003 Oct 15;23(28):9452-8. doi: 10.1523/JNEUROSCI.23-28-09452.2003. J Neurosci. 2003. PMID: 14561875 Free PMC article.
-
No requirement of alpha-internexin for nervous system development and for radial growth of axons.Brain Res Mol Brain Res. 1999 May 21;69(1):104-12. doi: 10.1016/s0169-328x(99)00104-7. Brain Res Mol Brain Res. 1999. PMID: 10350642
-
Neurofilaments in health and disease.Prog Nucleic Acid Res Mol Biol. 1998;61:1-23. doi: 10.1016/s0079-6603(08)60823-5. Prog Nucleic Acid Res Mol Biol. 1998. PMID: 9752717 Review.
-
Neurofilaments and Neurofilament Proteins in Health and Disease.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a018309. doi: 10.1101/cshperspect.a018309. Cold Spring Harb Perspect Biol. 2017. PMID: 28373358 Free PMC article. Review.
Cited by
-
Suppression of extensive neurofilament phosphorylation rescues α-Internexin/peripherin-overexpressing PC12 cells from neuronal cell death.PLoS One. 2012;7(8):e43883. doi: 10.1371/journal.pone.0043883. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952800 Free PMC article.
-
Shedding light on neurofilament involvement in cognitive decline in obstructive sleep apnea and its possible role as a biomarker.Front Psychiatry. 2023 Nov 30;14:1289367. doi: 10.3389/fpsyt.2023.1289367. eCollection 2023. Front Psychiatry. 2023. PMID: 38098628 Free PMC article. Review.
-
Neurofilament light interaction with GluN1 modulates neurotransmission and schizophrenia-associated behaviors.Transl Psychiatry. 2018 Aug 24;8(1):167. doi: 10.1038/s41398-018-0194-7. Transl Psychiatry. 2018. PMID: 30143609 Free PMC article.
-
The myosin Va head domain binds to the neurofilament-L rod and modulates endoplasmic reticulum (ER) content and distribution within axons.PLoS One. 2011 Feb 16;6(2):e17087. doi: 10.1371/journal.pone.0017087. PLoS One. 2011. PMID: 21359212 Free PMC article.
-
Global axonal transport rates are unaltered in htau mice in vivo.J Alzheimers Dis. 2013;37(3):579-86. doi: 10.3233/JAD-130671. J Alzheimers Dis. 2013. PMID: 23948900 Free PMC article.
References
-
- Al-Chalabi A, Miller CC. Neurofilaments and neurological disease. BioEssays. 2003;25:346–355. - PubMed
-
- Archer DR, Watson DF, Griffin JW. Phosphorylation-dependent immunoreactivity of neurofilaments and the rate of slow axonal transport in the central and peripheral axons of the rat dorsal root ganglion. J Neurochem. 1994;62:1119–1125. - PubMed
-
- Beaulieu JM, Robertson J, Julien JP. Interactions between peripherin and neurofilaments in cultured cells: disruption of peripherin assembly by the NF-M and NF-H subunits. Biochem Cell Biol. 1999;77:41–45. - PubMed
-
- Benson DL, Mandell JW, Shaw G, Banker G. Compartmentation of alpha-internexin and neurofilament triplet proteins in cultured hippocampal neurons. J Neurocytol. 1996;25:181–196. - PubMed
-
- Brown A. Visualization of single neurofilaments by immunofluorescence microscopy of splayed axonal cytoskeletons. Cell Motil Cytoskeleton. 1997;38:133–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
