Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates
- PMID: 17005870
- PMCID: PMC6674486
- DOI: 10.1523/JNEUROSCI.0896-06.2006
Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates
Abstract
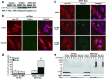
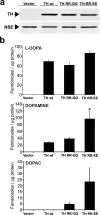
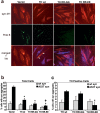
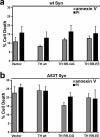
Aberrant aggregation of alpha-synuclein (alpha-syn) to form fibrils and insoluble aggregates has been implicated in the pathogenic processes of many neurodegenerative diseases. Despite the dramatic effects of dopamine in inhibiting the formation of alpha-syn fibrils by stabilization of oligomeric intermediates in cell-free systems, no studies have examined the effects of intracellular dopamine on alpha-syn aggregation. To study this process and its association with neurodegeneration, intracellular catechol levels were increased to various levels by expressing different forms of tyrosine hydroxylase, in cells induced to form alpha-syn aggregates. The increase in the steady-state dopamine levels inhibited the formation of alpha-syn aggregates and induced the formation of innocuous oligomeric intermediates. Analysis of transgenic mice expressing the disease-associated A53T mutant alpha-syn revealed the presence of oligomeric alpha-syn in nondegenerating dopaminergic neurons that do contain insoluble alpha-syn. These data indicate that intraneuronal dopamine levels can be a major modulator of alpha-syn aggregation and inclusion formation, with important implications on the selective degeneration of these neurons in Parkinson's disease.
Figures

Similar articles
-
Cellular oligomerization of alpha-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence.J Biol Chem. 2007 Oct 26;282(43):31621-30. doi: 10.1074/jbc.M704737200. Epub 2007 Sep 4. J Biol Chem. 2007. PMID: 17785456
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease.Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10813-8. doi: 10.1073/pnas.152339799. Epub 2002 Jul 16. Proc Natl Acad Sci U S A. 2002. PMID: 12122208 Free PMC article.
-
Alteration of Structure and Aggregation of α-Synuclein by Familial Parkinson's Disease Associated Mutations.Curr Protein Pept Sci. 2017;18(7):656-676. doi: 10.2174/1389203717666160314151706. Curr Protein Pept Sci. 2017. PMID: 26972727 Review.
-
Phase separation and other forms of α-Synuclein self-assemblies.Essays Biochem. 2022 Dec 16;66(7):987-1000. doi: 10.1042/EBC20220055. Essays Biochem. 2022. PMID: 36373662 Review.
Cited by
-
α-Synuclein in Parkinson's disease.Cold Spring Harb Perspect Med. 2012 Feb;2(2):a009399. doi: 10.1101/cshperspect.a009399. Cold Spring Harb Perspect Med. 2012. PMID: 22355802 Free PMC article. Review.
-
The interplay between lipids and dopamine on α-synuclein oligomerization and membrane binding.Biosci Rep. 2013 Oct 22;33(5):e00074. doi: 10.1042/BSR20130092. Biosci Rep. 2013. PMID: 24066973 Free PMC article.
-
Structural Features and Toxicity of α-Synuclein Oligomers Grown in the Presence of DOPAC.Int J Mol Sci. 2021 Jun 2;22(11):6008. doi: 10.3390/ijms22116008. Int J Mol Sci. 2021. PMID: 34199427 Free PMC article.
-
Leucine-rich repeat kinase 2 expression leads to aggresome formation that is not associated with alpha-synuclein inclusions.J Neuropathol Exp Neurol. 2009 Jul;68(7):785-96. doi: 10.1097/NEN.0b013e3181aaf4fd. J Neuropathol Exp Neurol. 2009. PMID: 19535993 Free PMC article.
-
Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies.Cell. 2011 Jul 8;146(1):37-52. doi: 10.1016/j.cell.2011.06.001. Epub 2011 Jun 23. Cell. 2011. PMID: 21700325 Free PMC article.
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Catillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Cabin DE, Shimazu K, Murphy DD, Cole NB, Gottschalk W, McIIwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. - PMC - PubMed
-
- Cappai R, Leck SL, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, Culvenor JG, Bottomley SP, Masters CL, Barnham KJ, Hill AF. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
