Furazolidone-based triple therapy for H pylori gastritis in children
- PMID: 17006997
- PMCID: PMC4088242
- DOI: 10.3748/wjg.v12.i34.5544
Furazolidone-based triple therapy for H pylori gastritis in children
Abstract
Aim: To evaluate the furazolidone-based triple therapy in children with symptomatic H pylori gastritis.
Methods: A prospective and consecutive open trial was carried out. The study included 38 patients with upper digestive symptoms sufficiently severe to warrant endoscopic investigation. H pylori status was defined based both on histology and on positive (13)C-urea breath test. Drug regimen was a seven-day course of omeprazole, clarithromycin and furazolidone (100 mg, 200 mg if over 30 kg) twice daily. Eradication of H pylori was assessed two months after treatment by histology and (13)C -urea breath test. Further clinical evaluation was performed 7 d, 2 and 6 mo after the treatment.
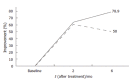
Results: Thirty-eight patients (24 females, 14 males) were included. Their age ranged from 4 to 17.8 (mean 10.9 +/- 3.7) years. On intent-to-treat analysis (n = 38), the eradication rate of H pylori was 73.7% (95% CI, 65.2%-82%) whereas in per-protocol analysis (n = 33) it was 84.8% (95% CI, 78.5%-91%). All the patients with duodenal ulcer (n = 7) were successfully treated (100% vs 56.2% with antral nodularity). Side effects were reported in 26 patients (68.4%), mainly vomiting (14/26) and abdominal pain (n = 13). Successfully treated dyspeptic patients showed improvement in 78.9% of H pylori-negative patients after six months and in 50% of H pylori-positive patients after six months of treatment.
Conclusion: Triple therapy with furazolidone achieves moderate efficacy in H pylori treatment. The eradication rate seems to be higher in patients with duodenal ulcer.
Figures
References
-
- Mégraud F, Lamouliatte H. Review article: the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1333–1343. - PubMed
-
- Wong WM, Xiao SD, Hu PJ, Wang WH, Gu Q, Huang JQ, Xia HH, Wu SM, Li CJ, Chen MH, et al. Standard treatment for Helicobacter pylori infection is suboptimal in non-ulcer dyspepsia compared with duodenal ulcer in Chinese. Aliment Pharmacol Ther. 2005;21:73–81. - PubMed
-
- Perez Aldana L, Kato M, Nakagawa S, Kawarasaki M, Nagasako T, Mizushima T, Oda H, Kodaira J, Shimizu Y, Komatsu Y, et al. The relationship between consumption of antimicrobial agents and the prevalence of primary Helicobacter pylori resistance. Helicobacter. 2002;7:306–309. - PubMed
-
- Larrosa-Haro A, Martínez-Puente EO, Coello-Ramírez P, Castillo de León YA, Bojórquez-Ramos Mdel C, Macías-Rosales R, García-Salazar O, Vázquez-Camacho G, Flores Márquez MR. [Efficacy of two Helicobacter pylori eradication treatments in children with recurrent abdominal pain] Rev Gastroenterol Mex. 2004;69:76–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical