Capillary electrophoresis separation in the presence of an immiscible boundary for droplet analysis
- PMID: 17007519
- PMCID: PMC2525566
- DOI: 10.1021/ac0613131
Capillary electrophoresis separation in the presence of an immiscible boundary for droplet analysis
Abstract
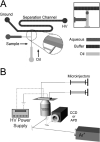
This paper demonstrates the ability to use capillary electrophoresis (CE) separation coupled with laser-induced fluorescence for analyzing the contents of single femtoliter-volume aqueous droplets. A single droplet was formed using a T-channel (3 microm wide by 3 microm tall) connected to microinjectors, and then the droplet was fluidically moved to an immiscible boundary that isolates the CE channel (50 microm wide by 50 microm tall) from the droplet generation region. Fusion of the aqueous droplet with the immiscible boundary effectively injects the droplet content into the separation channel. In addition to injecting the contents of droplets, we found aqueous samples can be introduced directly into the separation channel by reversibly penetrating and resealing the immiscible partition. Because droplet generation in channels requires hydrophobic surfaces, we have also investigated the advantages to using all hydrophobic channels versus channel systems with patterned hydrophobic and hydrophilic regions. To fabricate devices with patterned surface chemistry, we have developed a simple strategy based on differential wetting to deposit selectively a hydrophilic polymer (poly(styrenesulfonate)) onto desired regions of the microfluidic chip. Finally, we applied our device to the separation of a simple mixture of fluorescein-labeled amino acids contained within a approximately 10-fL droplet.
Figures




References
-
- Lorenz RM, Edgar JS, Jeffries GD, Chiu DT. Anal. Chem. 2006 In press. - PubMed
-
- Chiu DT. TrAC. 2003;22:528–536.
-
- He M, Edgar JS, Jeffries GD, Lorenz RM, Shelby JP, Chiu DT. Anal Chem. 2005;77:1539–1544. - PubMed
-
- Zheng B, Roach LS, Ismagilov RF. J. Am. Chem. Soc. 2003;125:11170–11171. - PubMed
-
- Dittrich PS, Jahnz M, Schwille P. Chembiochem. 2005;6:811–814. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

