A novel electron paramagnetic resonance-based assay for prostaglandin H synthase-1 activity
- PMID: 17007643
- PMCID: PMC1592475
- DOI: 10.1186/1476-9255-3-12
A novel electron paramagnetic resonance-based assay for prostaglandin H synthase-1 activity
Abstract
Background: Prostaglandin H2 synthase (PGHS) is the enzyme that catalyses the two-stage conversion of arachidonic acid to prostaglandin H2 (PGH2) prior to formation of prostanoids that are important in inflammation. PGHS isozymes (-1 and -2) are the target for nonsteroidal anti-inflammatory drugs (NSAIDs). Given the rekindled interest in specific anti-inflammatory PGHS inhibitors with reduced unwanted side effects, it is of paramount importance that there are reliable and efficient techniques to test new inhibitors. Here, we describe a novel in vitro electron paramagnetic resonance (EPR)-based assay for measuring the activity of PGHS-1.
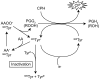
Methods: We validated a novel in vitro PGHS-1 activity assay based on the oxidation of spin-trap agent, 1-hydroxy-3-carboxy-pyrrolidine (CPH) to 3-carboxy-proxy (CP) under the action of the peroxidase element of PGHS-1. This quantifiable spin-adduct, CP, yields a characteristic 3-line electron paramagnetic (EPR) spectrum.
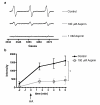
Results: The assay is simple, reproducible and facilitates rapid screening of inhibitors of PGHS-1. Aspirin (100 microM, 1 mM) caused significant inhibition of spin-adduct formation (72 +/- 11 and 100 +/- 16% inhibition of control respectively; P < 0.05). Indomethacin (100 microM) also abolished the signal (114 +/- 10% inhibition of control; P < 0.01). SA and the PGHS-2-selective inhibitor, NS398, failed to significantly inhibit spin-adduct generation (P > 0.05).
Conclusion: We have demonstrated and validated a simple, reproducible, quick and specific assay for detecting PGHS-1 activity and inhibition. The EPR-based assay described represents a novel approach to measuring PGHS activity and provides a viable and competitive alternative to existing assays.
Figures

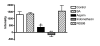
References
-
- Eling TE, Mason RP, Sivarajah K. The formation of aminopyrine cation radical by the peroxidase activity of prostaglandin H synthase and subsequent reactions of the radical. J Biol Chem. 1985;260:1601–1607. - PubMed
-
- Hoffmann C. COX-2 in brain and spinal cord implications for therapeutic use. Curr Med Chem. 2000;7:1113–1120. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

