Thioredoxin and ventricular remodeling
- PMID: 17007870
- PMCID: PMC1852508
- DOI: 10.1016/j.yjmcc.2006.08.006
Thioredoxin and ventricular remodeling
Abstract
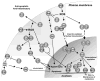
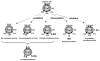
Increasing bodies of evidence indicate that reactive oxygen species (ROS) produced by mitochondria and other sources play an essential role in mediating ventricular remodeling after myocardial infarction and the development of heart failure. Antioxidants scavenge ROS, thereby maintaining the reduced environment of cells and inhibiting ventricular remodeling in the heart. Thioredoxin not only functions as a major antioxidant in the heart but also interacts with important signaling molecules and transcription factors, thereby modulating various cellular functions. The activity of thioredoxin is regulated by a variety of mechanisms, such as transcription, localization, protein-protein interaction, and post-translational modification. In this review, we will summarize the cardiac effects of thioredoxin and the mechanisms by which thioredoxin mediates inhibition of ventricular remodeling.
Figures
Similar articles
-
Modulation of signaling mechanisms in the heart by thioredoxin 1.Free Radic Biol Med. 2017 Aug;109:125-131. doi: 10.1016/j.freeradbiomed.2016.12.020. Epub 2016 Dec 16. Free Radic Biol Med. 2017. PMID: 27993729 Free PMC article. Review.
-
Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function.Circulation. 2015 Mar 24;131(12):1082-97. doi: 10.1161/CIRCULATIONAHA.114.012725. Epub 2015 Jan 27. Circulation. 2015. PMID: 25628390 Free PMC article.
-
Time course of hydrogen peroxide-thioredoxin balance and its influence on the intracellular signalling in myocardial infarction.Exp Physiol. 2012 Jun;97(6):741-9. doi: 10.1113/expphysiol.2012.064832. Epub 2012 Feb 24. Exp Physiol. 2012. PMID: 22366564
-
Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload.Circ Res. 2007 Dec 7;101(12):1328-38. doi: 10.1161/CIRCRESAHA.106.160515. Epub 2007 Oct 4. Circ Res. 2007. PMID: 17916779
-
Mechanistic Role of Thioredoxin 2 in Heart Failure.Adv Exp Med Biol. 2017;982:265-276. doi: 10.1007/978-3-319-55330-6_14. Adv Exp Med Biol. 2017. PMID: 28551792 Review.
Cited by
-
Thioredoxin-1 Protects Spinal Cord from Demyelination Induced by Methamphetamine through Suppressing Endoplasmic Reticulum Stress and Inflammation.Front Neurol. 2018 Feb 6;9:49. doi: 10.3389/fneur.2018.00049. eCollection 2018. Front Neurol. 2018. PMID: 29467717 Free PMC article.
-
Down-regulation of cardiac lineage protein (CLP-1) expression in CLP-1 +/- mice affords.J Cell Mol Med. 2009 Aug;13(8B):2744-2753. doi: 10.1111/j.1582-4934.2008.00404.x. J Cell Mol Med. 2009. PMID: 18624753 Free PMC article.
-
Vitamin D Supplementation Induces Cardiac Remodeling in Rats: Association with Thioredoxin-Interacting Protein and Thioredoxin.Arq Bras Cardiol. 2021 May;116(5):970-978. doi: 10.36660/abc.20190633. Arq Bras Cardiol. 2021. PMID: 34008824 Free PMC article. English, Portuguese.
-
Expression Profile of Circulating MicroRNAs in Dogs With Cardiac Hypertrophy: A Pilot Study.Front Vet Sci. 2021 Apr 9;8:652224. doi: 10.3389/fvets.2021.652224. eCollection 2021. Front Vet Sci. 2021. PMID: 33898546 Free PMC article.
-
Master redox regulator Trx1 upregulates SMYD1 & modulates lysine methylation.Biochim Biophys Acta. 2015 Dec;1854(12):1816-1822. doi: 10.1016/j.bbapap.2015.09.006. Epub 2015 Sep 26. Biochim Biophys Acta. 2015. PMID: 26410624 Free PMC article.
References
-
- Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. - PubMed
-
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–312. - PubMed
-
- Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78:3–14. - PubMed
-
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. - PubMed
-
- Yamawaki H, Berk BC. Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels. Curr Opin Nephrol Hypertens. 2005;14:149–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

