Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: Intrinsic membrane properties and responses to carbachol and orexins
- PMID: 17008019
- PMCID: PMC1775037
- DOI: 10.1016/j.neuroscience.2006.08.045
Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: Intrinsic membrane properties and responses to carbachol and orexins
Abstract
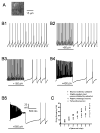
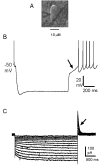
Pharmacological, lesion and single-unit recording techniques in several animal species have identified a region of the pontine reticular formation (subcoeruleus, SubC) just ventral to the locus coeruleus as critically involved in the generation of rapid-eye-movement (REM) sleep. However, the intrinsic membrane properties and responses of SubC neurons to neurotransmitters important in REM sleep control, such as acetylcholine and orexins/hypocretins, have not previously been examined in any animal species and thus were targeted in this study. We obtained whole-cell patch-clamp recordings from visually identified SubC neurons in rat brain slices in vitro. Two groups of large neurons (mean diameter 30 and 27 mum) were tentatively identified as cholinergic (rostral SubC) and noradrenergic (caudal SubC) neurons. SubC reticular neurons (non-cholinergic, non-noradrenergic) showed a medium-sized depolarizing sag during hyperpolarizing current pulses and often had a rebound depolarization (low-threshold spike, LTS). During depolarizing current pulses they exhibited little adaptation and fired maximally at 30-90 Hz. Those SubC reticular neurons excited by carbachol (n=27) fired spontaneously at 6 Hz, often exhibited a moderately sized LTS, and varied widely in size (17-42 mum). Carbachol-inhibited SubC reticular neurons were medium-sized (15-25 mum) and constituted two groups. The larger group (n=22) was silent at rest and possessed a prominent LTS and associated one to four action potentials. The second, smaller group (n=8) had a delayed return to baseline at the offset of hyperpolarizing pulses. Orexins excited both carbachol excited and carbachol inhibited SubC reticular neurons. SubC reticular neurons had intrinsic membrane properties and responses to carbachol similar to those described for other reticular neurons but a larger number of carbachol inhibited neurons were found (>50%), the majority of which demonstrated a prominent LTS and may correspond to pontine-geniculate-occipital burst neurons. Some or all carbachol-excited neurons are presumably REM-on neurons.
Figures





Similar articles
-
Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice.Eur J Neurosci. 2008 Jan;27(2):352-63. doi: 10.1111/j.1460-9568.2008.06024.x. Eur J Neurosci. 2008. PMID: 18215233 Free PMC article.
-
Effects of pedunculopontine nucleus (PPN) stimulation on caudal pontine reticular formation (PnC) neurons in vitro.J Neurophysiol. 2002 Jun;87(6):3033-47. doi: 10.1152/jn.2002.87.6.3033. J Neurophysiol. 2002. PMID: 12037206
-
The cholinergic agonist carbachol increases the frequency of spontaneous GABAergic synaptic currents in dorsal raphe serotonergic neurons in the mouse.Neuroscience. 2014 Jan 31;258:62-73. doi: 10.1016/j.neuroscience.2013.11.005. Epub 2013 Nov 11. Neuroscience. 2014. PMID: 24231737 Free PMC article.
-
Carbachol models of REM sleep: recent developments and new directions.Arch Ital Biol. 2001 Feb;139(1-2):147-68. Arch Ital Biol. 2001. PMID: 11256182 Review.
-
Pontine cholinergic mechanisms and their impact on respiratory regulation.Respir Physiol Neurobiol. 2004 Nov 15;143(2-3):235-49. doi: 10.1016/j.resp.2004.04.017. Respir Physiol Neurobiol. 2004. PMID: 15519558 Review.
Cited by
-
Carbachol excites sublaterodorsal nucleus neurons projecting to the spinal cord.J Physiol. 2014 Apr 1;592(7):1601-17. doi: 10.1113/jphysiol.2013.261800. Epub 2013 Dec 16. J Physiol. 2014. PMID: 24344163 Free PMC article.
-
Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats.Physiol Behav. 2012 Dec 5;107(5):726-32. doi: 10.1016/j.physbeh.2012.05.019. Epub 2012 May 28. Physiol Behav. 2012. PMID: 22652097 Free PMC article.
-
Recording Gamma Band Oscillations in Pedunculopontine Nucleus Neurons.J Vis Exp. 2016 Sep 14;(115):54685. doi: 10.3791/54685. J Vis Exp. 2016. PMID: 27684729 Free PMC article.
-
Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse.J Comp Neurol. 2013 Apr 15;521(6):1225-50. doi: 10.1002/cne.23290. J Comp Neurol. 2013. PMID: 23254904 Free PMC article.
-
Cholinergic modulation of GABAergic and glutamatergic transmission in the dorsal subcoeruleus: mechanisms for REM sleep control.Sleep. 2009 Sep;32(9):1135-47. doi: 10.1093/sleep/32.9.1135. Sleep. 2009. PMID: 19750918 Free PMC article.
References
-
- Amzica F, Steriade M. Progressive cortical synchronization of ponto-geniculo-occipital potentials during rapid eye movement sleep. Neuroscience. 1996;72:309–314. - PubMed
-
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. - PubMed
-
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. - PubMed
-
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

