Early anaerobic metabolisms
- PMID: 17008221
- PMCID: PMC1664682
- DOI: 10.1098/rstb.2006.1906
Early anaerobic metabolisms
Abstract
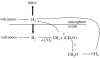
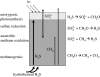
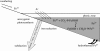
Before the advent of oxygenic photosynthesis, the biosphere was driven by anaerobic metabolisms. We catalogue and quantify the source strengths of the most probable electron donors and electron acceptors that would have been available to fuel early-Earth ecosystems. The most active ecosystems were probably driven by the cycling of H2 and Fe2+ through primary production conducted by anoxygenic phototrophs. Interesting and dynamic ecosystems would have also been driven by the microbial cycling of sulphur and nitrogen species, but their activity levels were probably not so great. Despite the diversity of potential early ecosystems, rates of primary production in the early-Earth anaerobic biosphere were probably well below those rates observed in the marine environment. We shift our attention to the Earth environment at 3.8Gyr ago, where the earliest marine sediments are preserved. We calculate, consistent with the carbon isotope record and other considerations of the carbon cycle, that marine rates of primary production at this time were probably an order of magnitude (or more) less than today. We conclude that the flux of reduced species to the Earth surface at this time may have been sufficient to drive anaerobic ecosystems of sufficient activity to be consistent with the carbon isotope record. Conversely, an ecosystem based on oxygenic photosynthesis was also possible with complete removal of the oxygen by reaction with reduced species from the mantle.
Figures
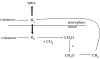


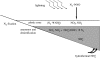
References
-
- Alperin M.J, Reeburgh W.S. Carbon and hydrogen isotope fractionation resulting from anaerobic methane oxidation. Global Biogeochem. Cycles. 1988;2:279–288.
-
- Alt J.C, Shanks W.C., III Serpentinization of abyssal peridotites from the MARK area, Mid-Atlantic Ridge: sulfur geochemistry and reaction modeling. Geochim. Cosmochim. Acta. 2003;67:641–653. doi:10.1016/S0016-7037(02)01142-0 - DOI
-
- Arthur M.A, Dean W.E. Organic-matter production and preservation and evolution of anoxia in the Holocene Black Sea. Paleoceanography. 1998;13:395–411. doi:10.1029/98PA01161 - DOI
-
- Baas Becking L.G.M. Studies on the sulphur bacteria. Ann. Bot. 1925;39:613–650.
-
- Bach W, Paulick H, Garrido C.J, Ildefonse B, Meurer W.P, Humphris S.E. Unraveling the sequence of serpentinization reactions: mineral chemistry, and petrophysics of serpentinites from MAR 15 °N (ODP Leg 209, Site 1274) Geophys. Res. Lett. 2006;33:L13306. doi:10.1029/2006GL025681 - DOI
Additional references
-
- Shen Y, Buick R, Canfield D.E. Isotopic evidence for microbial sulphate reduction in the early Archaean era. Nature. 2001;410:77–81. doi:10.1038/35065071 - DOI - PubMed
-
- Ueno Y, Yamada K, Yoshida N, Maruyama S, Isozaki Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature. 2006;440:516–519. doi:10.1038/nature04584 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
