Glycoprotein Ibalpha forms disulfide bonds with 2 glycoprotein Ibbeta subunits in the resting platelet
- PMID: 17008541
- PMCID: PMC1785083
- DOI: 10.1182/blood-2006-05-024091
Glycoprotein Ibalpha forms disulfide bonds with 2 glycoprotein Ibbeta subunits in the resting platelet
Abstract
It is widely accepted that glycoprotein (GP) Ib contains one Ibalpha and one Ibbeta subunit that are connected by a disulfide bond. It is unclear which Cys residue in Ibalpha, C484 or C485, forms the disulfide bond with Ibbeta. Using mutagenesis studies in transfected Chinese hamster ovary (CHO) cells, we found that both C484 and C485 formed a disulfide bond with C122 in Ibbeta. In the context of isolated peptides containing the Ibalpha or Ibbeta transmembrane domain and nearby Cys residue, C484 and C485 in the Ibalpha peptide were both capable of forming a disulfide bond with the Ibbeta peptide. Furthermore, coimmunoprecipitation of epitope-tagged subunits showed that at least 2 Ibbeta subunits but only 1 Ibalpha and 1 IX subunit were present in the GP Ib-IX complex. Finally, the size difference between GP Ib from transfected CHO cells and human platelets was attributed to a combination of sequence polymorphism and glycosylation difference in Ibalpha, not the number of Ibbeta subunits therein. Overall, these results demonstrate that Ibalpha is covalently connected to 2 Ibbeta subunits in the resting platelet, necessitating revision of the subunit stoichiometry of the GP Ib-IX-V complex. The alphabeta2 composition in GP Ib may provide the basis for possible disulfide rearrangement in the receptor complex.
Conflict of interest statement
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Figures

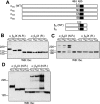

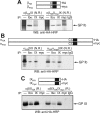


References
-
- Savage B, Shattil SJ, Ruggeri ZM. Modulation of platelet function through adhesion receptors: a dual role for glycoprotein IIb-IIIa (integrin αIIbβ3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J Biol Chem. 1992;267:11300–11306. - PubMed
-
- Dong JF, Li CQ, Sae-Tung G, Hyun W, Afshar-Kharghan V, Lopez JA. The cytoplasmic domain of glycoprotein (GP) Ibα constrains the lateral diffusion of the GP Ib-IX complex and modulates von Willebrand factor binding. Biochemistry. 1997;36:12421–12427. - PubMed
-
- Schade AJ, Arya M, Gao S, et al. Cytoplasmic truncation of glycoprotein Ibα weakens its interaction with von Willebrand factor and impairs cell adhesion. Biochemistry. 2003;42:2245–2251. - PubMed
-
- Englund GD, Bodnar RJ, Li Z, Ruggeri ZM, Du X. Regulation of von Willebrand factor binding to the platelet glycoprotein Ib-IX by a membrane skeleton-dependent inside-out signal. J Biol Chem. 2001;276:16952–16959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

