Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury
- PMID: 17008604
- PMCID: PMC2562953
- DOI: 10.1161/01.RES.0000247065.11756.19
Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury
Abstract
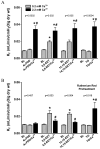
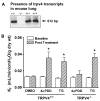
Disruption of the alveolar septal barrier leads to acute lung injury, patchy alveolar flooding, and hypoxemia. Although calcium entry into endothelial cells is critical for loss of barrier integrity, the cation channels involved in this process have not been identified. We hypothesized that activation of the vanilloid transient receptor potential channel TRPV4 disrupts the alveolar septal barrier. Expression of TRPV4 was confirmed via immunohistochemistry in the alveolar septal wall in human, rat, and mouse lung. In isolated rat lung, the TRPV4 activators 4alpha-phorbol-12,13-didecanoate and 5,6- or 14,15-epoxyeicosatrienoic acid, as well as thapsigargin, a known activator of calcium entry via store-operated channels, all increased lung endothelial permeability as assessed by measurement of the filtration coefficient, in a dose- and calcium-entry dependent manner. The TRPV antagonist ruthenium red blocked the permeability response to the TRPV4 agonists, but not to thapsigargin. Light and electron microscopy of rat and mouse lung revealed that TRPV4 agonists preferentially produced blebs or breaks in the endothelial and epithelial layers of the alveolar septal wall, whereas thapsigargin disrupted interendothelial junctions in extraalveolar vessels. The permeability response to 4alpha-phorbol-12,13-didecanoate was absent in TRPV4(-/-) mice, whereas the response to thapsigargin remained unchanged. Collectively, these findings implicate TRPV4 in disruption of the alveolar septal barrier and suggest its participation in the pathogenesis of acute lung injury.
Figures



Comment in
-
TRP channels and the regulation of vascular permeability: new insights from the lung microvasculature.Circ Res. 2006 Oct 27;99(9):915-7. doi: 10.1161/01.RES.0000249618.51440.c6. Circ Res. 2006. PMID: 17068297 No abstract available.
References
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
-
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. - PubMed
-
- Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353:2788–2796. - PubMed
-
- Moore TM, Chetham PM, Kelly JJ, Stevens T. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am J Physiol. 1998;275:L203–L222. - PubMed
-
- Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

