Trapping the tetrahedral intermediate in the alkaline phosphatase reaction by substitution of the active site serine with threonine
- PMID: 17008720
- PMCID: PMC2242381
- DOI: 10.1110/ps.062351506
Trapping the tetrahedral intermediate in the alkaline phosphatase reaction by substitution of the active site serine with threonine
Abstract
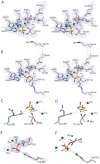
We report here the construction of a mutant version of Escherichia coli alkaline phosphatase (AP) in which the active site Ser was replaced by Thr (S102T), in order to investigate whether the enzyme can utilize Thr as the nucleophile and whether the rates of the critical steps in the mechanism are altered by the substitution. The mutant AP with Thr at position 102 exhibited an approximately 4000-fold decrease in k(cat) along with a small decrease in Km. The decrease in catalytic efficiency of approximately 2000-fold was a much smaller drop than that observed when Ala or Gly were substituted at position 102. The mechanism by which Thr can substitute for Ser in AP was further investigated by determining the X-ray structure of the S102T enzyme in the presence of the Pi (S102T_Pi), and after soaking the crystals with substrate (S102T_sub). In the S102T_Pi structure, the Pi was coordinated differently with its position shifted by 1.3 A compared to the structure of the wild-type enzyme in the presence of Pi. In the S102T_sub structure, a covalent Thr-Pi intermediate was observed, instead of the expected bound substrate. The stereochemistry of the phosphorus in the S102T_sub structure was inverted compared to the stereochemistry in the wild-type structure, as would be expected after the first step of a double in-line displacement mechanism. We conclude that the S102T mutation resulted in a shift in the rate-determining step in the mechanism allowing us to trap the covalent intermediate of the reaction in the crystal.
Figures
Similar articles
-
Kinetic and X-ray structural studies of three mutant E. coli alkaline phosphatases: insights into the catalytic mechanism without the nucleophile Ser102.J Mol Biol. 1998 Apr 3;277(3):647-62. doi: 10.1006/jmbi.1998.1635. J Mol Biol. 1998. PMID: 9533886
-
Artificial evolution of an enzyme active site: structural studies of three highly active mutants of Escherichia coli alkaline phosphatase.J Mol Biol. 2002 Mar 1;316(4):941-53. doi: 10.1006/jmbi.2001.5384. J Mol Biol. 2002. PMID: 11884134
-
Metal specificity is correlated with two crucial active site residues in Escherichia coli alkaline phosphatase.Biochemistry. 2005 Jun 14;44(23):8378-86. doi: 10.1021/bi050155p. Biochemistry. 2005. PMID: 15938627
-
The mechanism of the alkaline phosphatase reaction: insights from NMR, crystallography and site-specific mutagenesis.FEBS Lett. 1999 Nov 26;462(1-2):7-11. doi: 10.1016/s0014-5793(99)01448-9. FEBS Lett. 1999. PMID: 10580082 Review.
-
Structure and mechanism of alkaline phosphatase.Annu Rev Biophys Biomol Struct. 1992;21:441-83. doi: 10.1146/annurev.bb.21.060192.002301. Annu Rev Biophys Biomol Struct. 1992. PMID: 1525473 Review.
Cited by
-
Structural and Functional Integration of Tissue-Nonspecific Alkaline Phosphatase Within the Alkaline Phosphatase Superfamily: Evolutionary Insights and Functional Implications.Metabolites. 2024 Nov 25;14(12):659. doi: 10.3390/metabo14120659. Metabolites. 2024. PMID: 39728440 Free PMC article. Review.
-
Cellular function and molecular structure of ecto-nucleotidases.Purinergic Signal. 2012 Sep;8(3):437-502. doi: 10.1007/s11302-012-9309-4. Epub 2012 May 4. Purinergic Signal. 2012. PMID: 22555564 Free PMC article. Review.
-
High-resolution analysis of Zn(2+) coordination in the alkaline phosphatase superfamily by EXAFS and x-ray crystallography.J Mol Biol. 2012 Jan 6;415(1):102-17. doi: 10.1016/j.jmb.2011.10.040. Epub 2011 Oct 28. J Mol Biol. 2012. PMID: 22056344 Free PMC article.
-
Divergence of chemical function in the alkaline phosphatase superfamily: structure and mechanism of the P-C bond cleaving enzyme phosphonoacetate hydrolase.Biochemistry. 2011 May 3;50(17):3481-94. doi: 10.1021/bi200165h. Epub 2011 Apr 8. Biochemistry. 2011. PMID: 21366328 Free PMC article.
-
Essential Functional Interplay of the Catalytic Groups in Acid Phosphatase.ACS Catal. 2022 Mar 18;12(6):3357-3370. doi: 10.1021/acscatal.1c05656. Epub 2022 Feb 28. ACS Catal. 2022. PMID: 35356705 Free PMC article.
References
-
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. - PubMed
-
- Brunger, A.T., Adams, P.D., Rice, L.M. 1997. New applications of simulated annealing in X-ray crystallography and solution NMR. Structure 5: 325–336. - PubMed
-
- Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S. et al. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54: 905–921. - PubMed
-
- Chaidaroglou, A., Brezinski, J.D., Middleton, S.A., Kantrowitz, E.R. 1988. Function of arginine in the active site of Escherichia coli alkaline phosphatase. Biochemistry 27: 8338–8343. - PubMed
-
- Chen, L., Neidhart, D., Kohlbrenner, W.M., Mandecki, W., Bell, S., Sowadski, J., Abad-Zapatero, C. 1992. 3-D structure of a mutant (Asp101 → Ser) of E. coli alkaline phosphatase with higher catalytic activity. Protein Eng. 5: 605–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

