Induction of physiological thermotolerance in MDCK monolayers: contribution of heat shock protein 70
- PMID: 17009600
- PMCID: PMC1576477
- DOI: 10.1379/csc-194r.1
Induction of physiological thermotolerance in MDCK monolayers: contribution of heat shock protein 70
Abstract
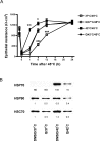
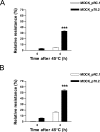
Enhanced survival of both individual cells and whole organisms following a heat stress is termed thermotolerance. In organisms, the maintenance of tissue function rather than the survival of individual cells ultimately determines outcome following thermal challenge. We used MDCK kidney epithelial cells to compare alterations in chaperone activity (as a measure of cellular tolerance) and epithelial barrier function (as a measure of physiological tolerance) after thermal challenge. Quercetin, an inhibitor of heat shock factor-dependent transcriptional activity, both potentiated the effects of heat on naive monolayers and blocked conditioning of monolayers following moderate heat shock, suggesting a central role of heat shock protein (HSP) family members in the maintenance of epithelial integrity. We used MDCK cells that constitutively overexpressed HSP70 to demonstrate 2 functionally distinct components of the response of monolayers to thermal stress. The maintenance of epithelial barrier function during exposure to elevated temperatures is regulated by a complex network of processes that involve the actions of HSP70 but that are independent of alterations in chaperone activity as reflected by changes in the thermal inactivation/refolding of luciferase. In contrast, the restoration of barrier function following a heat stress is directly modulated by HSP70 in a manner that can be fully accounted for by changes in chaperone activity. This study demonstrates an important, albeit complex, protective role for heat shock proteins in the modulation of MDCK epithelial barrier function following a thermal stress.
Figures


Similar articles
-
Lack of cross-tolerance following heat and cadmium exposure in functional MDCK monolayers.J Appl Toxicol. 2008 Oct;28(7):885-94. doi: 10.1002/jat.1352. J Appl Toxicol. 2008. PMID: 18418844
-
In vivo chaperone activity of heat shock protein 70 and thermotolerance.Mol Cell Biol. 1999 Mar;19(3):2069-79. doi: 10.1128/MCB.19.3.2069. Mol Cell Biol. 1999. PMID: 10022894 Free PMC article.
-
Expression of Hsp70 and Hsp27 in lens epithelial cells in contused eye of rat modulated by thermotolerance or quercetin.Mol Vis. 2006 May 9;12:445-50. Mol Vis. 2006. PMID: 16710168
-
Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia.Int J Hyperthermia. 1995 Jul-Aug;11(4):459-88. doi: 10.3109/02656739509022483. Int J Hyperthermia. 1995. PMID: 7594802 Review.
-
Regulation of thermotolerance and ischemic tolerance.EXS. 1996;77:467-81. doi: 10.1007/978-3-0348-9088-5_31. EXS. 1996. PMID: 8856991 Review.
Cited by
-
Heat acclimation-induced intracellular HSP70 in humans: a meta-analysis.Cell Stress Chaperones. 2020 Jan;25(1):35-45. doi: 10.1007/s12192-019-01059-y. Epub 2019 Dec 10. Cell Stress Chaperones. 2020. PMID: 31823288 Free PMC article. Review.
-
Reduced Hsp70 and Glutamine in Pediatric Severe Malaria Anemia: Role of Hemozoin in Suppressing Hsp70 and NF-κB activation.Mol Med. 2016 Oct;22:570-584. doi: 10.2119/molmed.2016.00130. Epub 2016 Aug 30. Mol Med. 2016. PMID: 27579474 Free PMC article.
-
Specific TLR-mediated HSP70 activation plays a potential role in host defense against the intestinal parasite Giardia duodenalis.Front Microbiol. 2023 Mar 2;14:1120048. doi: 10.3389/fmicb.2023.1120048. eCollection 2023. Front Microbiol. 2023. PMID: 36937289 Free PMC article.
-
The Gastrointestinal Exertional Heat Stroke Paradigm: Pathophysiology, Assessment, Severity, Aetiology and Nutritional Countermeasures.Nutrients. 2020 Feb 19;12(2):537. doi: 10.3390/nu12020537. Nutrients. 2020. PMID: 32093001 Free PMC article. Review.
-
Age-progressive interplay of HSP-proteostasis, ECM-cell junctions and biomechanics ensures C. elegans astroglial architecture.Nat Commun. 2024 Apr 3;15(1):2861. doi: 10.1038/s41467-024-46827-2. Nat Commun. 2024. PMID: 38570505 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
