Release of sequestered malaria parasites upon injection of a glycosaminoglycan
- PMID: 17009869
- PMCID: PMC1579244
- DOI: 10.1371/journal.ppat.0020100
Release of sequestered malaria parasites upon injection of a glycosaminoglycan
Abstract
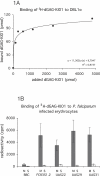
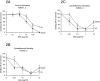
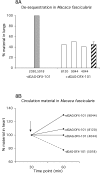
Severe human malaria is attributable to an excessive sequestration of Plasmodium falciparum-infected and uninfected erythrocytes in vital organs. Strains of P. falciparum that form rosettes and employ heparan sulfate as a host receptor are associated with development of severe forms of malaria. Heparin, which is similar to heparan sulfate in that it is composed of the same building blocks, was previously used in the treatment of severe malaria, but it was discontinued due to the occurrence of serious side effects such as intracranial bleedings. Here we report to have depolymerized heparin by periodate treatment to generate novel glycans (dGAG) that lack anticoagulant-activity. The dGAGs disrupt rosettes, inhibit merozoite invasion of erythrocytes and endothelial binding of P. falciparum-infected erythrocytes in vitro, and reduce sequestration in in vivo models of severe malaria. An intravenous injection of dGAGs blocks up to 80% of infected erythrocytes from binding in the micro-vasculature of the rat and releases already sequestered parasites into circulation. P. falciparum-infected human erythrocytes that sequester in the non-human primate Macaca fascicularis were similarly found to be released in to the circulation upon a single injection of 500 mug of dGAG. We suggest dGAGs to be promising candidates for adjunct therapy in severe malaria.
Conflict of interest statement
Figures





References
-
- Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, et al. Human cerebral malaria: Association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. - PubMed
-
- Roberts DJ, Pain A, Kai O, Kortok M, Marsh K. Autoagglutination of malaria-infected red blood cells and malaria severity. Lancet. 2000;355:1427–1428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

