Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers
- PMID: 17009875
- PMCID: PMC1584263
- DOI: 10.1371/journal.pgen.0020156
Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers
Abstract
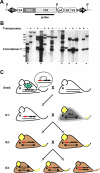
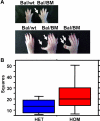
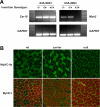
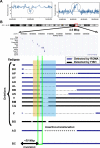
Previous studies of the Sleeping Beauty (SB) transposon system, as an insertional mutagen in the germline of mice, have used reverse genetic approaches. These studies have led to its proposed use for regional saturation mutagenesis by taking a forward-genetic approach. Thus, we used the SB system to mutate a region of mouse Chromosome 11 in a forward-genetic screen for recessive lethal and viable phenotypes. This work represents the first reported use of an insertional mutagen in a phenotype-driven approach. The phenotype-driven approach was successful in both recovering visible and behavioral mutants, including dominant limb and recessive behavioral phenotypes, and allowing for the rapid identification of candidate gene disruptions. In addition, a high frequency of recessive lethal mutations arose as a result of genomic rearrangements near the site of transposition, resulting from transposon mobilization. The results suggest that the SB system could be used in a forward-genetic approach to recover interesting phenotypes, but that local chromosomal rearrangements should be anticipated in conjunction with single-copy, local transposon insertions in chromosomes. Additionally, these mice may serve as a model for chromosome rearrangements caused by transposable elements during the evolution of vertebrate genomes.
Conflict of interest statement
Competing interests. DAL is a founding partner in Discovery Genomics, Inc. (DGI), a biotechnology company pursuing the use of SB and other transposable elements for gene therapy. None of the work described involved DGI.
Figures




References
-
- Dupuy AJ, Fritz S, Largaespada DA. Transposition and gene disruption in the male germline of the mouse. Genesis. 2001;30:82–88. - PubMed
-
- Keng VW, Yae K, Hayakawa T, Mizuno S, Uno Y, et al. Region-specific saturation germline mutagenesis in mice using the Sleeping Beauty transposon system. Nat Methods. 2005;2:763–769. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

