Functional expression of a single-chain antibody to ErbB-2 in plants and cell-free systems
- PMID: 17010186
- PMCID: PMC1592514
- DOI: 10.1186/1479-5876-4-39
Functional expression of a single-chain antibody to ErbB-2 in plants and cell-free systems
Abstract
Background: Aberrant signaling by ErbB-2 (HER 2, Neu), a member of the human Epidermal Growth Factor (EGF) receptor family, is associated with an aggressive clinical behaviour of carcinomas, particularly breast tumors. Antibodies targeting the ErbB-2 pathway are a preferred therapeutic option for patients with advanced breast cancer, but a worldwide deficit in the manufacturing capacities of mammalian cell bioreactors is foreseen.
Methods: Herein, we describe a multi-platform approach for the production of recombinant Single chain Fragments of antibody variable regions (ScFvs) to ErbB-2 that involves their functional expression in (a) bacteria, (b) transient as well as stable transgenic tobacco plants, and (c) a newly developed cell-free transcription-translation system.
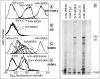
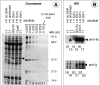
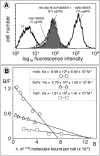
Results: An ScFv (ScFv800E6) was selected by cloning immunoglobulin sequences from murine hybridomas, and was expressed and fully functional in all the expression platforms, thereby representing the first ScFv to ErbB-2 produced in hosts other than bacteria and yeast. ScFv800E6 was optimized with respect to redox synthesis conditions. Different tags were introduced flanking the ScFv800E6 backbone, with and without spacer arms, including a novel Strep II tag that outperforms conventional streptavidin-based detection systems. ScFv800E6 was resistant to standard chemical radiolabeling procedures (i.e. Chloramine T), displayed a binding ability extremely similar to that of the parental monovalent Fab' fragment, as well as a flow cytometry performance and an equilibrium binding affinity (Ka approximately 2 x 10(8) M(-1)) only slightly lower than those of the parental bivalent antibody, suggesting that its binding site is conserved as compared to that of the parental antibody molecule. ScFv800E6 was found to be compatible with routine reagents for immunohistochemical staining.
Conclusion: ScFv800E6 is a useful reagent for in vitro biochemical and immunodiagnostic applications in oncology, and a candidate for future in vivo studies.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

