Role of streptococcal T antigens in superficial skin infection
- PMID: 17012387
- PMCID: PMC1797348
- DOI: 10.1128/JB.01179-06
Role of streptococcal T antigens in superficial skin infection
Abstract
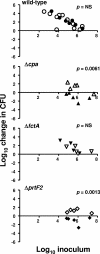
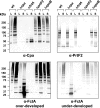
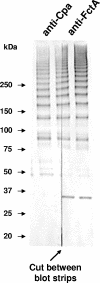
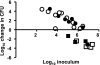
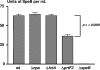
FCT region genes of Streptococcus pyogenes encode surface proteins that include fibronectin- and collagen-binding proteins and the serological markers known as T antigens, some of which give rise to pilus-like appendages. It remains to be established whether FCT region surface proteins contribute to virulence by in vivo models of infection. In this study, a highly sensitive and ecologically relevant humanized mouse model was used to measure superficial skin infection. Three genes encoding FCT region surface proteins essential for T-serotype specificity were inactivated. Both the Deltacpa and DeltaprtF2 mutants were highly attenuated for virulence when topically applied to the skin following exponential growth but were fully virulent when delivered in stationary phase. In contrast, the DeltafctA mutant was virulent at the skin, regardless of its initial growth state. Immunoblots of cell extracts revealed anti-FctA-reactive, ladder-like polymers characteristic of streptococcal pili. In addition, FctA formed a heteropolymer with the putative collagen-binding protein Cpa. The DeltafctA mutant showed a loss in anti-Cpa-reactive polymers, whereas anti-FctA-reactive polymers were reduced in the Deltacpa mutant. The findings suggest that both FctA and Cpa are required for pilus formation, but importantly, an intact pilus is not essential for Cpa-mediated virulence. Although it is an integral part of the T-antigen complex, the fibronectin-binding protein PrtF2 is not covalently linked to the FctA- and Cpa-containing heteropolymer derived from cell extracts. The data provide direct evidence that streptococcal T antigens function as virulence factors in vivo, but they also reveal that a pilus-like structure is not essential for the most common form of streptococcal skin disease.
Figures

Similar articles
-
Mode of expression and functional characterization of FCT-3 pilus region-encoded proteins in Streptococcus pyogenes serotype M49.Infect Immun. 2009 Jan;77(1):32-44. doi: 10.1128/IAI.00772-08. Epub 2008 Oct 13. Infect Immun. 2009. PMID: 18852238 Free PMC article.
-
The small regulatory RNA FasX enhances group A Streptococcus virulence and inhibits pilus expression via serotype-specific targets.Mol Microbiol. 2015 Apr;96(2):249-62. doi: 10.1111/mmi.12935. Epub 2015 Feb 4. Mol Microbiol. 2015. PMID: 25586884 Free PMC article.
-
The Group A Streptococcus serotype M2 pilus plays a role in host cell adhesion and immune evasion.Mol Microbiol. 2017 Jan;103(2):282-298. doi: 10.1111/mmi.13556. Epub 2016 Nov 14. Mol Microbiol. 2017. PMID: 27741558
-
Group A streptococcal infections of the skin: molecular advances but limited therapeutic progress.Curr Opin Infect Dis. 2006 Apr;19(2):132-8. doi: 10.1097/01.qco.0000216623.82950.11. Curr Opin Infect Dis. 2006. PMID: 16514337 Review.
-
Genetics, Structure, and Function of Group A Streptococcal Pili.Front Microbiol. 2021 Feb 9;12:616508. doi: 10.3389/fmicb.2021.616508. eCollection 2021. Front Microbiol. 2021. PMID: 33633705 Free PMC article. Review.
Cited by
-
Ultra-deep sequencing of Hadza hunter-gatherers recovers vanishing gut microbes.Cell. 2023 Jul 6;186(14):3111-3124.e13. doi: 10.1016/j.cell.2023.05.046. Epub 2023 Jun 21. Cell. 2023. PMID: 37348505 Free PMC article.
-
Identification and Characterization of Serotype-Specific Variation in Group A Streptococcus Pilus Expression.Infect Immun. 2018 Jan 22;86(2):e00792-17. doi: 10.1128/IAI.00792-17. Print 2018 Feb. Infect Immun. 2018. PMID: 29158432 Free PMC article.
-
Flexible architecture of the Streptococcus pyogenes FCT genome region: finally the clue for understanding purulent skin diseases and long-term persistence?J Bacteriol. 2007 Feb;189(4):1181-4. doi: 10.1128/JB.01748-06. Epub 2006 Dec 1. J Bacteriol. 2007. PMID: 17142401 Free PMC article. No abstract available.
-
Genomic differences between sequence types 1 and 104 of Streptococcus suis Serotype 2.PeerJ. 2022 Oct 6;10:e14144. doi: 10.7717/peerj.14144. eCollection 2022. PeerJ. 2022. PMID: 36221266 Free PMC article.
-
Group A Streptococcus Pili-Roles in Pathogenesis and Potential for Vaccine Development.Microorganisms. 2024 Mar 11;12(3):555. doi: 10.3390/microorganisms12030555. Microorganisms. 2024. PMID: 38543606 Free PMC article. Review.
References
-
- Barocchi, M. A., J. Ries, X. Zogaj, C. Hemsley, B. Albiger, A. Kanth, S. Dahlberg, J. Fernebro, M. Moschioni, V. Masignani, K. Hultenby, A. R. Taddei, K. Beiter, F. Wartha, A. von Euler, A. Covacci, D. W. Holden, S. Normark, R. Rappuoli, and B. Henriques-Normark. 2006. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. USA 103:2857-2862. - PMC - PubMed
-
- Bessen, D. E., J. R. Carapetis, B. Beall, R. Katz, M. Hibble, B. J. Currie, T. Collingridge, M. W. Izzo, D. A. Scaramuzzino, and K. S. Sriprakash. 2000. Contrasting molecular epidemiology of group A streptococci causing tropical and non-tropical infections of the skin and throat. J. Infect. Dis. 182:1109-1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

