Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment
- PMID: 17012587
- PMCID: PMC1694257
- DOI: 10.1128/AEM.01065-06
Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment
Abstract
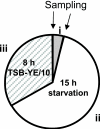
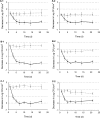
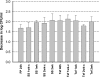
The objective of this study was to evaluate the resistance of biofilms of Listeria monocytogenes to sanitizing agents under laboratory conditions simulating a food processing environment. Biofilms were initially formed on stainless steel and Teflon coupons using a five-strain mixture of L. monocytogenes. The coupons were then subjected to repeated 24-h daily cycles. Each cycle consisted of three sequential steps: (i) a brief (60 s) exposure of the coupons to a sanitizing agent (a mixture of peroxides) or saline as a control treatment, (ii) storage of the coupons in sterile plastic tubes without any nutrients or water for 15 h, (iii) and incubation of the coupons in diluted growth medium for 8 h. This regimen was repeated daily for up to 3 weeks and was designed to represent stresses encountered by bacteria in a food processing environment. The bacteria on the coupons were reduced in number during the first week of the simulated food processing (SFP) regimen, but then adapted to the stressful conditions and increased in number. Biofilms repeatedly exposed the peroxide sanitizer in the SFP regimen developed resistance to the peroxide sanitizer as well as other sanitizers (quaternary ammonium compounds and chlorine). Interestingly, cells that were removed from the biofilms on peroxide-treated and control coupons were not significantly different in their resistance to sanitizing agents. These data suggest that the resistance of the treated biofilms to sanitizing agents may be due to attributes of extracellular polymeric substances and is not an intrinsic attribute of the cells in the biofilm.
Figures
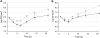

References
-
- Blackman, I. C., and J. F. Frank. 1996. Growth of Listeria monocytogenes as a biofilm on various food-processing surfaces. J. Food Prot. 59:827-831. - PubMed
-
- Boyd, A., and A. M. Chakrabarty. 1995. Pseudomonas aerugenosa biofilms—role of the alginate exopolysaccharide. J. Ind. Microbiol. 15:162-168. - PubMed
-
- Bremer, P. J., I. Monk, and R. Butler. 2002. Inactivation of Listeria monocytogenes/Flavobacterium spp. biofilms using chlorine: impact of substrate, pH, time and concentration. Lett. Appl. Microbiol. 35:321-325. - PubMed
-
- Briandet, R., V. Leriche, B. Carpentier, and M. Bellon-Fontaine. 1999. Effects of the growth procedure on the surface hydrophobicity of Listeria monocytogenes cells and their adhesion to stainless steel. J. Food Prot. 62:994-998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

