Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock
- PMID: 17012604
- PMCID: PMC1626623
- DOI: 10.1105/tpc.105.037358
Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock
Abstract
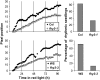
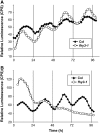
Circadian gating of light signaling limits the timing of maximum responsiveness to light to specific times of day. The fhy3 (for far-red elongated hypocotyl3) mutant of Arabidopsis thaliana is involved in independently gating signaling from a group of photoreceptors to an individual response. fhy3 shows an enhanced response to red light during seedling deetiolation. Analysis of two independent fhy3 alleles links enhanced inhibition of hypocotyl elongation in response to red light with an arrhythmic pattern of hypocotyl elongation. Both alleles also show disrupted rhythmicity of central-clock and clock-output gene expression in constant red light. fhy3 exhibits aberrant phase advances under red light pulses during the subjective day. Release-from-light experiments demonstrate clock disruption in fhy3 during the early part of the subjective day in constant red light, suggesting that FHY3 is important in gating red light signaling for clock resetting. The FHY3 gating function appears crucial in the early part of the day for the maintenance of rhythmicity under these conditions. However, unlike previously described Arabidopsis gating mutants that gate all light signaling, gating of direct red light-induced gene expression in fhy3 is unaffected. FHY3 appears to be a novel gating factor, specifically in gating red light signaling to the clock during daytime.
Figures






References
-
- Alabadi, D., Oyama, T., Yanovsky, M.J., Harmon, F.G., Más, P., and Kay, S.A. (2001). Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293 880–883. - PubMed
-
- Cashmore, A.R. (2005). Cryptochromes. In Photomorphogenesis in Plants and Bacteria, E. Schafer and F. Nagy, eds (Dordrecht, The Netherlands: Springer), pp. 379–406.
-
- Devlin, P.F. (2002). Signs of the time: Environmental input to the circadian clock. J. Exp. Bot. 53 1535–1550. - PubMed
-
- Devlin, P.F. (2006). Circadian regulation of photomorphogenesis. In Photomorphogenesis in Plants and Bacteria, E. Schafer and F. Nagy, eds (Dordrecht, The Netherlands: Springer), pp. 567–604.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

