Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression
- PMID: 17013391
- PMCID: PMC1797066
- DOI: 10.1038/ni1392
Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression
Abstract
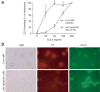
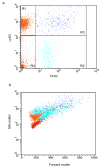
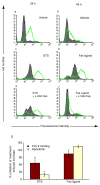
During the resolution phase of inflammation, the 'corpses' of apoptotic leukocytes are gradually cleared by macrophages. Here we report that during the resolution of peritonitis, the CCR5 chemokine receptor ligands CCL3 and CCL5 persisted in CCR5-deficient mice. CCR5 expression on apoptotic neutrophils and activated apoptotic T cells sequestered and effectively cleared CCL3 and CCL5 from sites of inflammation. CCR5 expression on late apoptotic human polymorphonuclear cells was downregulated by proinflammatory stimuli, including tumor necrosis factor, and was upregulated by 'proresolution' lipid mediators, including lipoxin A4, resolvin E1 and protectin D1. Our results suggest that CCR5+ apoptotic leukocytes act as 'terminators' of chemokine signaling during the resolution of inflammation.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests (see the
Figures
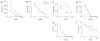
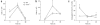

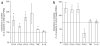
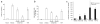




References
-
- Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. Oxford University Press: New York; 2004.
-
- Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins. 1997;53:107–137. - PubMed
-
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. - PubMed
-
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. - PubMed
-
- Rossi AG, Haslett C. In: Proinflammatory and Antiinflammatory Peptides. Said SI, editor. Marcel Dekker; New York: 1998. pp. 9–24.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

