Neurotrophin-3 ameliorates sensory-motor deficits in Er81-deficient mice
- PMID: 17013886
- PMCID: PMC2587023
- DOI: 10.1002/dvdy.20964
Neurotrophin-3 ameliorates sensory-motor deficits in Er81-deficient mice
Abstract
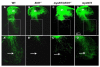
Two factors, the ETS transcription factor ER81 and skeletal muscle-derived neurotrophin-3 (NT3), are essential for the formation of muscle spindles and the function of spindle afferent-motoneuron synapses in the spinal cord. Spindles either degenerate completely or are abnormal, and spindle afferents fail to project to spinal motoneurons in Er81 null mice; however, the interactions between ER81 and NT3 during the processes of afferent neuron and muscle spindle development are poorly understood. To examine if overexpression of NT3 in muscle rescues spindles and afferent-motoneuron connectivity in the absence of ER81, we generated myoNT3;Er81(-/-) double-mutant mice that selectively overexpress NT3 in muscle in the absence of ER81. Spindle reflex arcs in myoNT3;Er81(-/-) mutants differed greatly from Er81 null mice. Muscle spindle densities were greater and more afferents projected into the ventral spinal cord in myoNT3;Er81(-/-) mice. Spindles of myoNT3;Er81(-/-) muscles responded normally to repetitive muscle taps, and the monosynaptic inputs from Ia afferents to motoneurons, grossly reduced in Er81(-/-) mutants, were restored to wild-type levels in myoNT3;Er81(-/-) mice. Thus, an excess of muscle-derived NT3 reverses deficits in spindle numbers and afferent function induced by the absence of ER81. We conclude that muscle-derived NT3 can modulate spindle density and afferent-motoneuron connectivity independently of ER81.
(c) 2006 Wiley-Liss, Inc.
Figures






References
-
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. - PubMed
-
- Bradbury E, Khemani S, King VR, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neuro-sci. 1999;11:3873–3883. - PubMed
-
- Castellani V, Bolz J. Opposing roles for neurotrophin-3 in targeting and collateral formation of distinct sets of developing cortical neurons. Development. 1999;126:3335–3345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

