Characterization of neutralizing epitopes within the major capsid protein of human papillomavirus type 33
- PMID: 17014700
- PMCID: PMC1599721
- DOI: 10.1186/1743-422X-3-83
Characterization of neutralizing epitopes within the major capsid protein of human papillomavirus type 33
Abstract
Background: Infections with papillomaviruses induce type-specific immune responses, mainly directed against the major capsid protein, L1. Based on the propensity of the L1 protein to self-assemble into virus-like particles (VLPs), type-specific vaccines have already been developed. In order to generate vaccines that target a broader spectrum of HPV types, extended knowledge of neutralizing epitopes is required. Despite the association of human papillomavirus type 33 (HPV33) with cervical carcinomas, fine mapping of neutralizing conformational epitopes on HPV33 has not been reported yet. By loop swapping between HPV33 and HPV16 capsid proteins, we have identified amino acid sequences critical for the binding of conformation-dependent type-specific neutralizing antibodies to surface-exposed hyper variable loops of HPV33 capsid protein L1.
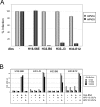
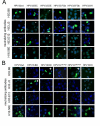
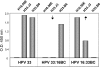
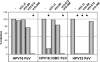
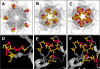
Results: Reactivities of monoclonal antibodies (mAbs) H33.B6, H33.E12, H33.J3 and H16.56E with HPV16:33 and HPV33:16 hybrid L1 VLPs revealed the complex structures of their conformational epitopes as well as the major residues contributing to their binding sites. Whereas the epitope of mAb H33.J3 was determined by amino acids (aa) 51-58 in the BC loop of HPV33 L1, sequences of at least two hyper variable loops, DE (aa 132-140) and FGb (aa 282-291), were found to be essential for binding of H33.B6. The epitope of H33.E12 was even more complex, requiring sequences of the FGa loop (aa 260-270), in addition to loops DE and FGb.
Conclusion: These data demonstrate that neutralizing epitopes in HPV33 L1 are mainly located on the tip of the capsomere and that several hyper variable loops contribute to form these conformational epitopes. Knowledge of the antigenic structure of HPV is crucial for designing hybrid particles as a basis for intertypic HPV vaccines.
Figures

Similar articles
-
HPV11 mutant virus-like particles elicit immune responses that neutralize virus and delineate a novel neutralizing domain.Virology. 2000 Jan 20;266(2):237-45. doi: 10.1006/viro.1999.0083. Virology. 2000. PMID: 10639310
-
Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region.Virology. 2007 Feb 20;358(2):266-72. doi: 10.1016/j.virol.2006.08.037. Epub 2006 Sep 28. Virology. 2007. PMID: 17010405
-
Disassembly and reassembly of human papillomavirus virus-like particles produces more virion-like antibody reactivity.Virol J. 2012 Feb 22;9:52. doi: 10.1186/1743-422X-9-52. Virol J. 2012. PMID: 22356831 Free PMC article.
-
Chapter 12: Prophylactic HPV vaccines: underlying mechanisms.Vaccine. 2006 Aug 31;24 Suppl 3:S3/106-13. doi: 10.1016/j.vaccine.2006.05.110. Epub 2006 Jun 23. Vaccine. 2006. PMID: 16949996 Review.
-
Papillomavirus virus-like particles as vehicles for the delivery of epitopes or genes.Arch Virol. 2006 Nov;151(11):2133-48. doi: 10.1007/s00705-006-0798-8. Epub 2006 Jun 22. Arch Virol. 2006. PMID: 16791442 Review.
Cited by
-
Immunogenicity correlation in cynomolgus monkeys between Luminex-based total IgG immunoassay and pseudovirion-based neutralization assay for a 14-valent recombinant human papillomavirus vaccine.J Med Virol. 2022 Aug;94(8):3946-3955. doi: 10.1002/jmv.27763. Epub 2022 Apr 21. J Med Virol. 2022. PMID: 35388509 Free PMC article.
-
Multiple heparan sulfate binding site engagements are required for the infectious entry of human papillomavirus type 16.J Virol. 2013 Nov;87(21):11426-37. doi: 10.1128/JVI.01721-13. Epub 2013 Aug 21. J Virol. 2013. PMID: 23966387 Free PMC article.
-
Structural basis for the shared neutralization mechanism of three classes of human papillomavirus type 58 antibodies with disparate modes of binding.J Virol. 2021 Mar 10;95(7):e01587-20. doi: 10.1128/JVI.01587-20. Epub 2021 Jan 20. J Virol. 2021. PMID: 33472937 Free PMC article.
-
Efficacy of the AS04-Adjuvanted HPV16/18 Vaccine: Pooled Analysis of the Costa Rica Vaccine and PATRICIA Randomized Controlled Trials.J Natl Cancer Inst. 2020 Aug 1;112(8):818-828. doi: 10.1093/jnci/djz222. J Natl Cancer Inst. 2020. PMID: 31697384 Free PMC article.
-
The possible regions to design Human Papilloma Viruses vaccine in Iranian L1 protein.Biologia (Bratisl). 2020;75(5):749-759. doi: 10.2478/s11756-019-00386-w. Epub 2019 Dec 24. Biologia (Bratisl). 2020. PMID: 32435064 Free PMC article.
References
-
- Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, Kurman RJ. Human papillomavirus infection of the cervix: Relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992;79:328–337. - PubMed
-
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, de Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

