Study protocol: delayed intervention randomised controlled trial within the Medical Research Council (MRC) Framework to assess the effectiveness of a new palliative care service
- PMID: 17014714
- PMCID: PMC1615868
- DOI: 10.1186/1472-684X-5-7
Study protocol: delayed intervention randomised controlled trial within the Medical Research Council (MRC) Framework to assess the effectiveness of a new palliative care service
Abstract
Background: Palliative care has been proposed to help meet the needs of patients who suffer progressive non-cancer conditions but there have been few evaluations of service development initiatives. We report here a novel protocol for the evaluation of a new palliative care service in this context.
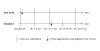
Methods/design: Using the MRC Framework for the Evaluation of Complex Interventions we modelled a new palliative care and neurology service for patients severely affected by Multiple Sclerosis (MS). We conducted qualitative interviews with patients, families and staff, plus a literature review to model and pilot the service. Then we designed a delayed intervention randomised controlled trial to test its effectiveness as part of phase II of the MRC framework. Inclusion criteria for the trial were patients identified by referring clinicians as having unresolved symptoms or psychological concerns. Referrers were advised to use a score of greater than 8 on the Expanded Disability Scale was a benchmark. Consenting patients newly referred to the new service were randomised to either receive the palliative care service immediately (fast-track) or after a 12-week wait (standard best practice). Face to face interviews were conducted at baseline (before intervention), and at 4-6, 10-12 (before intervention for the standard-practice group), 16-18 and 22-24 weeks with patients and their carers using standard questionnaires to assess symptoms, palliative care outcomes, function, service use and open comments. Ethics committee approval was granted separately for the qualitative phase and then for the trial.
Discussion: We publish the protocol trial here, to allow methods to be reviewed in advance of publication of the results. The MRC Framework for the Evaluation of Complex Interventions was helpful in both the design of the service, methods for evaluation in convincing staff and the ethics committee to accept the trial. The research will provide valuable information on the effects of palliative care among non-cancer patients and a method to evaluate palliative care in this context.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical