Differential expression of WNT4 in testicular and ovarian development in a marsupial
- PMID: 17014734
- PMCID: PMC1609105
- DOI: 10.1186/1471-213X-6-44
Differential expression of WNT4 in testicular and ovarian development in a marsupial
Abstract
Background: WNT4 is a key regulator of gonadal differentiation in humans and mice, playing a pivotal role in early embryogenesis. Using a marsupial, the tammar wallaby, in which most gonadal differentiation occurs after birth whilst the young is in the pouch, we show by quantitative PCR during early testicular and ovarian development that WNT4 is differentially expressed in gonads.
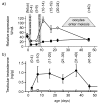
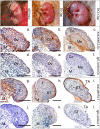
Results: Before birth, WNT4 mRNA expression was similar in indifferent gonads of both sexes. After birth, in females WNT4 mRNA dramatically increased during ovarian differentiation, reaching a peak by day 9-13 post partum (pp) when the ovarian cortex and medulla are first distinguishable. WNT4 protein was localised in the ovarian cortex and at the medullary boundary. WNT4 mRNA then steadily decreased to day 49, by which time all the female germ cells have entered meiotic arrest. In males, WNT4 mRNA was down-regulated in testes immediately after birth, coincident with the time that seminiferous cords normally form, and rose gradually after day 8. By day 49, when testicular androgen production normally declines, WNT4 protein was restricted to the Leydig cells.
Conclusion: This is the first localisation of WNT4 protein in developing gonads and is consistent with a role for WNT4 in steroidogenesis. Our data provide strong support for the suggestion that WNT4 not only functions as an anti-testis gene during early development, but is also necessary for later ovarian and testicular function.
Figures


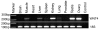


Similar articles
-
Marsupial WT1 has a novel isoform and is expressed in both somatic and germ cells in the developing ovary and testis.Sex Dev. 2007;1(3):169-80. doi: 10.1159/000102106. Sex Dev. 2007. PMID: 18391528
-
[Cloning, expression and characterization of a gene encoding signal transduction protein Wnt4 of Schistosoma japonicum].Sheng Wu Gong Cheng Xue Bao. 2007 May;23(3):392-7. Sheng Wu Gong Cheng Xue Bao. 2007. PMID: 17577981 Chinese.
-
A microarray analysis of the XX Wnt4 mutant gonad targeted at the identification of genes involved in testis vascular differentiation.Gene Expr Patterns. 2007 Jan;7(1-2):82-92. doi: 10.1016/j.modgep.2006.05.012. Epub 2006 Jun 6. Gene Expr Patterns. 2007. Corrected and republished in: Gene Expr Patterns. 2008 Sep;8(7-8):529-37. doi: 10.1016/j.gep.2008.05.006. PMID: 16844427 Corrected and republished.
-
WNT4 and sex development.Sex Dev. 2008;2(4-5):210-8. doi: 10.1159/000152037. Epub 2008 Nov 5. Sex Dev. 2008. PMID: 18987495 Review.
-
Balancing the bipotential gonad between alternative organ fates: a new perspective on an old problem.Dev Dyn. 2006 Sep;235(9):2292-300. doi: 10.1002/dvdy.20894. Dev Dyn. 2006. PMID: 16881057 Review.
Cited by
-
Physical map of two tammar wallaby chromosomes: a strategy for mapping in non-model mammals.Chromosome Res. 2008;16(8):1159-75. doi: 10.1007/s10577-008-1266-y. Epub 2008 Nov 8. Chromosome Res. 2008. PMID: 18987984
-
R-spondin1 and FOXL2 act into two distinct cellular types during goat ovarian differentiation.BMC Dev Biol. 2008 Apr 2;8:36. doi: 10.1186/1471-213X-8-36. BMC Dev Biol. 2008. PMID: 18384673 Free PMC article.
-
Genetics and timing of sex determination in the East African cichlid fish Astatotilapia burtoni.BMC Genet. 2014 Dec 14;15:140. doi: 10.1186/s12863-014-0140-5. BMC Genet. 2014. PMID: 25494637 Free PMC article.
-
The expression of Wnt4 is regulated by estrogen via an estrogen receptor alpha-dependent pathway in rat pituitary growth hormone-producing cells.Acta Histochem Cytochem. 2009 Dec 29;42(6):205-13. doi: 10.1267/ahc.09033. Epub 2009 Dec 22. Acta Histochem Cytochem. 2009. PMID: 20126574 Free PMC article.
-
Molecular cloning and sexually dimorphic expression of wnt4 in olive flounder (Paralichthys olivaceus).Fish Physiol Biochem. 2016 Aug;42(4):1167-76. doi: 10.1007/s10695-016-0206-6. Epub 2016 Feb 26. Fish Physiol Biochem. 2016. PMID: 26920537
References
-
- Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol. 2002;282:F431–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

