Co-activator p120 is increased by gonadotropins in the rat ovary and enhances progesterone receptor activity
- PMID: 17014737
- PMCID: PMC1617106
- DOI: 10.1186/1477-7827-4-50
Co-activator p120 is increased by gonadotropins in the rat ovary and enhances progesterone receptor activity
Abstract
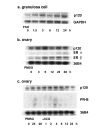
Background: Ovarian follicular development is primarily dependent on pituitary gonadotropins. Identification of gonadotropin-inducible genes in the ovary is one of the effective approaches for the study of follicular development. In this study we identify rat homologue of p120, a nuclear transcription co-activator, as one of the FSH inducible genes in the rat granulosa cells.
Methods: A full-length cDNA encoding rat p120 was cloned, and expression of the gene in the ovary was examined by Northern blotting. Tissue localization of p120 was examined by in situ hybridization. Cellular functions of p120 were studied by co-transfection of rat p120 gene together with estrogen receptor (ER)-alpha, ER-beta, androgen receptor (AR), or progesterone receptor (PR) genes.
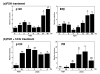
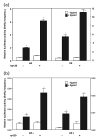
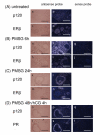
Results: A full-length cDNA encoding rat p120 was characterized as a protein with 957 amino acid residues. Rat p120 was expressed ubiquitously, but strongly in the ovary and the testis. Expression of p120 mRNA was also induced in vivo by PMSG or PMSG/hCG treatment. Strong expression of p120 mRNA was observed in the granulosa cells of pre-ovulatory large antral follicles. Progesterone receptor was co-localized with p120 in the large antral follicles. Co-transfection experiments revealed that rat p120 activated AR, ER-alpha, ER-beta, and PR in the presence of their respective ligands.
Conclusion: These observations suggest that rat p120 is strongly induced in the ovarian granulosa cells, and may work together with PR in the granulosa cells of ovulatory follicles to promote the ovulation process.
Figures


Similar articles
-
Estrogen receptor-beta mRNA expression in rat ovary: down-regulation by gonadotropins.Mol Endocrinol. 1997 Feb;11(2):172-82. doi: 10.1210/mend.11.2.9887. Mol Endocrinol. 1997. PMID: 9013764
-
Gonadotropin regulation of RIP140 messenger ribonucleic acid expression in the rat ovary.Life Sci. 2007 Sep 1;81(12):1003-8. doi: 10.1016/j.lfs.2007.07.027. Epub 2007 Aug 10. Life Sci. 2007. PMID: 17850828
-
Oestrogen receptor alpha and beta, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep.Reproduction. 2006 Jan;131(1):81-92. doi: 10.1530/rep.1.00704. Reproduction. 2006. PMID: 16388012
-
Progesterone receptors: expression and regulation in the mammalian ovary.Clin Obstet Gynecol. 1996 Jun;39(2):424-35. doi: 10.1097/00003081-199606000-00016. Clin Obstet Gynecol. 1996. PMID: 8734007 Review.
-
Multiplicity of progesterone's actions and receptors in the mammalian ovary.Biol Reprod. 2006 Jul;75(1):2-8. doi: 10.1095/biolreprod.105.049924. Epub 2006 Feb 1. Biol Reprod. 2006. PMID: 16452458 Review.
Cited by
-
Control of oocyte release by progesterone receptor-regulated gene expression.Nucl Recept Signal. 2009 Dec 31;7:e012. doi: 10.1621/nrs.07012. Nucl Recept Signal. 2009. PMID: 20087433 Free PMC article. Review.
-
Understanding the role of BRD8 in human carcinogenesis.Cancer Sci. 2024 Sep;115(9):2862-2870. doi: 10.1111/cas.16263. Epub 2024 Jul 5. Cancer Sci. 2024. PMID: 38965933 Free PMC article. Review.
-
Extracorporeal Shock Wave Therapy Combined with Platelet-Rich Plasma during Preventive and Therapeutic Stages of Intrauterine Adhesion in a Rat Model.Biomedicines. 2022 Feb 17;10(2):476. doi: 10.3390/biomedicines10020476. Biomedicines. 2022. PMID: 35203684 Free PMC article.
References
-
- Richards JS, Fitzpatrick SL, Clemens JW, Morris JK, Alliston T, Sirois J. Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res. 1995;50:223–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

