N-cadherin is required for neural crest remodeling of the cardiac outflow tract
- PMID: 17014840
- PMCID: PMC1866362
- DOI: 10.1016/j.ydbio.2006.09.003
N-cadherin is required for neural crest remodeling of the cardiac outflow tract
Abstract
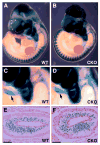
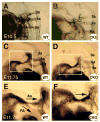
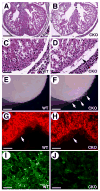
Cardiac neural crest cells undergo extensive cell rearrangements during the formation of the aorticopulmonary septum in the outflow tract. However, the morphogenetic mechanisms involved in this fundamental process remain poorly understood. To determine the function of the Ca2+-dependent cell adhesion molecule, N-cadherin, in murine neural crest, we applied the Cre/loxP system and created mouse embryos genetically mosaic for N-cadherin. Specifically, deletion of N-cadherin in neural crest cells led to embryonic lethality with distinct cardiovascular defects. Neural crest cell migration and homing to the cardiac outflow tract niche were unaffected by loss of N-cadherin. However, N-cadherin-deficient neural crest cells were unable to undergo the normal morphogenetic changes associated with outflow tract remodeling, resulting in persistent truncus arteriosus in the majority of mutant embryos. Other mutant embryos initiated aorticopulmonary septum formation; however, the neural crest cells were unable to elongate and align properly along the midline and remained rounded with limited contact with their neighbors. Interestingly, rotation of the outflow tract was incomplete in these mutants suggesting that alignment of the channels is dependent on N-cadherin-generated cytoskeletal forces. A second cardiac phenotype was observed where loss of N-cadherin in the epicardium led to disruption of heterotypic cell interactions between the epicardium and myocardium resulting in a thinned ventricular myocardium. Thus, we conclude that in addition to its role in myocardial cell adhesion, N-cadherin is required for neural crest cell rearrangements critical for patterning of the cardiac outflow tract and in the maintenance of epicardial-myocardial cell interactions.
Figures





References
-
- Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. - PubMed
-
- Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–60. - PubMed
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. - PubMed
-
- Bronner-Fraser M, Wolf JJ, Murray BA. Effects of antibodies against N-cadherin and N-CAM on the cranial neural crest and neural tube. Dev Biol. 1992;153:291–301. - PubMed
-
- Chen TH, Chang TC, Kang JO, Choudhary B, Makita T, Tran CM, Burch JB, Eid H, Sucov HM. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev Biol. 2002;250:198–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

