A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization
- PMID: 17015431
- PMCID: PMC1578695
- DOI: 10.1101/gad.1447006
A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization
Abstract
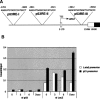
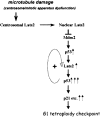
Damage to the mitotic spindle and centrosome dysfunction can lead to cancer. To prevent this, cells trigger a succession of checkpoint responses, where an initial mitotic delay is followed by slippage without cytokinesis, spawning tetraploid G1 cells that undergo a p53-dependent G1/S arrest. We describe the importance of Lats2 (Large Tumor Suppressor 2) in this checkpoint response. Lats2 binds Mdm2, inhibits its E3 ligase activity, and activates p53. Nocodazole, a microtubule poison that provokes centrosome/mitotic apparatus dysfunction, induces Lats2 translocation from centrosomes to the nucleus and p53 accumulation. In turn, p53 rapidly and selectively up-regulates Lats2 expression in G2/M cells, thereby defining a positive feedback loop. Abrogation of Lats2 promotes accumulation of polyploid cells upon exposure to nocodazole, which can be prevented by direct activation of p53. The Lats2-Mdm2-p53 axis thus constitutes a novel checkpoint pathway critical for the maintenance of proper chromosome number.
Figures



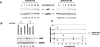

References
-
- Abe, Y., Ohsugi, M., Haraguchi, K., Fujimoto, J., Yamamoto, T. LATS2–Ajuba complex regulates γ-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 2006;580:782–788. - PubMed
-
- Andreassen, P.R., Lohez, O.D., Margolis, R.L. G2 and spindle assembly checkpoint adaptation, and tetraploidy arrest: Implications for intrinsic and chemically induced genomic instability. Mutat. Res. 2003;532:245–253. - PubMed
-
- Aronheim, A. Membrane recruitment systems for analysis of protein–protein interactions. Methods Mol. Biol. 2001;177:319–328. - PubMed
-
- Barr, F.A., Sillje, H.H., Nigg, E.A. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 2004;5:429–440. - PubMed
-
- Bond, G.L., Hu, W., Levine, A.J. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr. Cancer Drug Targets. 2005;5:3–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
