Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni
- PMID: 17015453
- PMCID: PMC1698078
- DOI: 10.1128/IAI.00958-06
Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni
Abstract
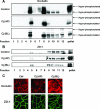
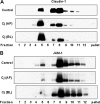
Campylobacter jejuni is a leading cause of human enterocolitis and is associated with postinfectious complications, including irritable bowel syndrome and Guillain-Barré syndrome. However, the pathogenesis of C. jejuni infection remains poorly understood. Paracellular pathways in intestinal epithelial cells are gated by intercellular junctions (tight junctions and adherens junctions), providing a functional barrier between luminal microbes and host immune cells in the lamina propria. Here we describe alterations in tight junctions in intestinal epithelial monolayers following C. jejuni infection. Apical infection of polarized T84 monolayers caused a time-dependent decrease in transepithelial electrical resistance (TER). Immunofluorescence microscopy revealed a redistribution of the tight junctional transmembrane protein occludin from an intercellular to an intracellular location. Subcellular fractionation using equilibrium sucrose density gradients demonstrated decreased hyperphosphorylated occludin in lipid rafts, Triton X-100-soluble fractions, and the Triton X-100-insoluble pellet following apical infection. Apical infection with C. jejuni also caused rapid activation of NF-kappaB and AP-1, phosphorylation of extracellular signal-regulated kinase, Jun N-terminal protein kinase, and p38 mitogen-activated protein kinases, and basolateral secretion of the CXC chemokine interleukin-8 (IL-8). Basolateral infection with C. jejuni caused a more rapid decrease in TER, comparable redistribution of tight-junction proteins, and secretion of more IL-8 than that seen with apical infection. These results suggest that compromised barrier function and increased chemokine expression contribute to the pathogenesis of C. jejuni-induced enterocolitis.
Figures



References
-
- Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 174:4453-4460. - PubMed
-
- Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. - PubMed
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

