Mutation in the Trapalpha/Ssr1 gene, encoding translocon-associated protein alpha, results in outflow tract morphogenetic defects
- PMID: 17015483
- PMCID: PMC1636874
- DOI: 10.1128/MCB.00913-06
Mutation in the Trapalpha/Ssr1 gene, encoding translocon-associated protein alpha, results in outflow tract morphogenetic defects
Abstract
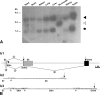
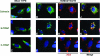
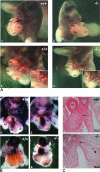
Translocon-associated protein complex (TRAP) is thought to be required for efficient protein-specific translocation across the endoplasmic reticulum membrane. We created a mutation in the Trapalpha gene that leads to the synthesis of a truncated TRAPalpha protein fused to ShBle-beta-galactosidase. Analysis of Trapalpha cDNAs reveals that among three different messenger RNAs expressed in the mouse, one of them encodes a slightly larger protein that differs in its C-terminal end. This mRNA, specific for skeletal muscle and heart, is only expressed after birth. Homozygous Trapalpha mutant pups die at birth, likely as a result of severe cardiac defects. Indeed, the septation of the proximal part of the outflow tract is absent, resulting in a double-outlet right ventricle. Studies of protein secretion in transfected embryonic fibroblasts reveal that the TRAP complex does not function properly in homozygous mutant cells and confirm, in vivo, the involvement of TRAP in substrate-specific translocation. Our results provide the first in vivo demonstration that a member of the TRAP complex plays a crucial role in mammalian heart development and suggest that TRAPalpha could be involved in translocation of factors necessary for maturation of endocardial cushions.
Figures


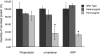


Similar articles
-
Translocon-associated protein subunit Trap-γ/Ssr3 is required for vascular network formation in the mouse placenta.Dev Dyn. 2011 Feb;240(2):394-403. doi: 10.1002/dvdy.22528. Epub 2011 Jan 11. Dev Dyn. 2011. PMID: 21246656
-
Deficient endoplasmic reticulum translocon-associated protein complex limits the biosynthesis of proinsulin and insulin.FASEB J. 2021 May;35(5):e21515. doi: 10.1096/fj.202002774R. FASEB J. 2021. PMID: 33811688 Free PMC article.
-
Translocon-associated protein TRAP delta and a novel TRAP-like protein are coordinately expressed with pro-opiomelanocortin in Xenopus intermediate pituitary.Biochem J. 1995 Nov 15;312 ( Pt 1)(Pt 1):205-13. doi: 10.1042/bj3120205. Biochem J. 1995. PMID: 7492314 Free PMC article.
-
Understanding the mammalian TRAP complex function(s).Open Biol. 2020 May;10(5):190244. doi: 10.1098/rsob.190244. Epub 2020 May 27. Open Biol. 2020. PMID: 32453970 Free PMC article. Review.
-
The expanding role of the ER translocon in membrane protein folding.J Cell Biol. 2007 Dec 31;179(7):1333-5. doi: 10.1083/jcb.200711107. J Cell Biol. 2007. PMID: 18166647 Free PMC article. Review.
Cited by
-
Exploring SSR1 as a novel diagnostic and prognostic biomarker in hepatocellular carcinoma, and its relationship with immune infiltration.Transl Cancer Res. 2024 Oct 31;13(10):5278-5299. doi: 10.21037/tcr-24-277. Epub 2024 Oct 29. Transl Cancer Res. 2024. PMID: 39525035 Free PMC article.
-
Requirement for translocon-associated protein (TRAP) α in insulin biogenesis.Sci Adv. 2019 Dec 4;5(12):eaax0292. doi: 10.1126/sciadv.aax0292. eCollection 2019 Dec. Sci Adv. 2019. PMID: 31840061 Free PMC article.
-
The concept of translocational regulation.J Cell Biol. 2008 Jul 28;182(2):225-32. doi: 10.1083/jcb.200804157. Epub 2008 Jul 21. J Cell Biol. 2008. PMID: 18644895 Free PMC article. Review.
-
Cytosolic quality control of mislocalized proteins requires RNF126 recruitment to Bag6.Mol Cell. 2014 Jul 17;55(2):227-37. doi: 10.1016/j.molcel.2014.05.025. Epub 2014 Jun 26. Mol Cell. 2014. PMID: 24981174 Free PMC article.
-
A cytoplasmic negative regulator isoform of ATF7 impairs ATF7 and ATF2 phosphorylation and transcriptional activity.PLoS One. 2011;6(8):e23351. doi: 10.1371/journal.pone.0023351. Epub 2011 Aug 16. PLoS One. 2011. PMID: 21858082 Free PMC article.
References
-
- Bartram, U., D. G. M. Molin, L. J. Wisse, A. Mohamad, L. P. Sandford, T. Doestchman, C. P. Speer, R. E. Poelmann, and A. C. Gittenberger-de Groot. 2001. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGFbeta(2)-knockout mice. Circulation 103:2745-2752. - PubMed
-
- Beckmann, R., D. Bubeck, R. Grassucci, P. Penczek, A. Verschoor, G. Blobel, and J. Frank. 1997. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science 278:2123-2126. - PubMed
-
- Brown, C. B., L. Feiner, M. M. Lu, J. Li, X. Ma, A. L. Webber, L. Jia, J. A. Raper, and J. A. Epstein. 2001. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 128:3071-3080. - PubMed
-
- Camus, A., C. Kress, C. Babinet, and J. Barra. 1996. Unexpected behavior of a gene trap vector comprising a fusion between the Sh ble and lacZ genes. Mol. Reprod. Dev. 45:255-263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
