Kidney failure in mice lacking the tetraspanin CD151
- PMID: 17015618
- PMCID: PMC2064491
- DOI: 10.1083/jcb.200603073
Kidney failure in mice lacking the tetraspanin CD151
Abstract
The tetraspanin CD151 is a cell-surface molecule known for its strong lateral interaction with the laminin-binding integrin alpha3beta1. Patients with a nonsense mutation in CD151 display end-stage kidney failure associated with regional skin blistering and sensorineural deafness, and mice lacking the integrin alpha3 subunit die neonatally because of severe abnormalities in the lung and kidney epithelia. We report the generation of Cd151-null mice that recapitulate the renal pathology of human patients, i.e., with age they develop massive proteinuria caused by focal glomerulosclerosis, disorganization of the glomerular basement membrane, and tubular cystic dilation. However, neither skin integrity nor hearing ability are impaired in the Cd151-null mice. Furthermore, we generated podocyte-specific conditional knockout mice for the integrin alpha3 subunit that show renal defects similar to those in the Cd151 knockout mice. Our results support the hypothesis that CD151 plays a key role in strengthening alpha3beta1-mediated adhesion in podocytes.
Figures
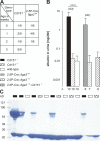
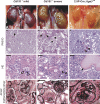
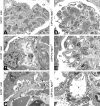

References
-
- Assad, L., M.M. Schwartz, I. Virtanen, and V.E. Gould. 1993. Immunolocalization of tenascin and cellular fibronectins in diverse glomerulopathies. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 63:307–316. - PubMed
-
- Borradori, L., and A. Sonnenberg. 1999. Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Invest. Dermatol. 112:411–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

