Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells
- PMID: 17015620
- PMCID: PMC2064505
- DOI: 10.1083/jcb.200605012
Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells
Abstract
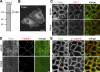
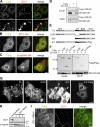
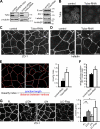
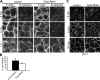
Epithelial cells are typically arranged in a honeycomb-like pattern, minimizing their cell-cell contact areas, which suggests that some tension operates for shaping of the cell boundaries. However, the molecular mechanisms that generate such tension remain unknown. We found that Tuba, which is a Cdc42-specific GEF, was concentrated at the apical-most region of cell junctions in simple epithelia via its interaction with ZO-1. RNAi-mediated depletion of Tuba altered the geometrical configuration of cell junctions, resulting in a curved and slack appearance. At the subcellular level, Tuba inactivation modified the assembly pattern of junctional F-actin and E-cadherin. Tuba RNAi also retarded cell junction formation in calcium-switch experiments. Suppression of Cdc42 activity or depletion of N-WASP, which is an effector of Cdc42, mimicked the effects of Tuba depletion. Conversely, overexpression of dominant-active Cdc42 or N-WASP enhanced the junction formation of Tuba-depleted cells. These results suggest that Tuba controls the shaping of cell junctions through the local activation of Cdc42 and its effectors.
Figures



References
-
- Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271:20246–20249. - PubMed
-
- Ando-Akatsuka, Y., S. Yonemura, M. Itoh, M. Furuse, and S. Tsukita. 1999. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell. Physiol. 179:115–125. - PubMed
-
- Benais-Pont, G., A. Punn, C. Flores-Maldonado, J. Eckert, G. Raposo, T.P. Fleming, M. Cereijido, M.S. Balda, and K. Matter. 2003. Identification of a tight junction–associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J. Cell Biol. 160:729–740. - PMC - PubMed
-
- Blanchoin, L., K.J. Amann, H.N. Higgs, J.B. Marchand, D.A. Kaiser, and T.D. Pollard. 2000. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 404:1007–1011. - PubMed
-
- Bokoch, G.M., B.P. Bohl, and T.H. Chuang. 1994. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J. Biol. Chem. 269:31674–31679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

