Bactericidal activity of both secreted and nonsecreted microcin E492 requires the mannose permease
- PMID: 17015644
- PMCID: PMC1636244
- DOI: 10.1128/JB.00688-06
Bactericidal activity of both secreted and nonsecreted microcin E492 requires the mannose permease
Abstract
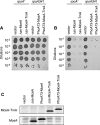
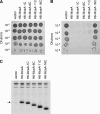
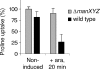
Microcin E492 (MccE492) is a bactericidal protein secreted by Klebsiella pneumoniae that is active against various species of Enterobacteriaceae. Interaction of MccE492 with target cells leads to the depolarization and permeabilization of their inner membranes. Several MccE492-specific proteins are required for the maturation and secretion of active MccE492. Surprisingly, the expression of only MceA, the polypeptide backbone of MccE492, is shown here to be toxic by itself. We refer to this phenomenon as endogenous MceA bactericidal activity to differentiate it from the action of extracellularly secreted MccE492. The toxicity of endogenous MceA is enhanced by an efficient targeting to the inner membrane. However, a periplasmic intermediate state is not required for MceA toxicity. Indeed, endogenous MceA remains fully active when it is fused to thioredoxin-1, a fast-folding protein that promotes retention of the C terminus of MceA in the cytoplasm. The C-terminal domain of MccE492 is required only for delivery from the extracellular environment to the periplasm, and it is not required for inner membrane damage. A common component is absolutely essential for the bactericidal activity of both endogenous MceA and extracellular MccE492. Indeed, toxicity is strictly dependent on the presence of ManYZ, an inner membrane protein complex involved in mannose uptake. Based on these findings, we propose a new model for cell entry, inner membrane insertion, and toxic activity of MccE492.
Figures





Comment in
-
The mannose transporter complex: an open door for the macromolecular invasion of bacteria.J Bacteriol. 2006 Oct;188(20):7036-8. doi: 10.1128/JB.01074-06. J Bacteriol. 2006. PMID: 17015642 Free PMC article. No abstract available.
References
-
- Belin, D., L. M. Guzman, S. Bost, M. Konakova, F. Silva, and J. Beckwith. 2004. Functional activity of eukaryotic signal sequences in Escherichia coli: the ovalbumin family of serine protease inhibitors. J. Mol. Biol. 335:437-453. - PubMed
-
- Bieler, S., L. Estrada, R. Lagos, M. Baeza, J. Castilla, and C. Soto. 2005. Amyloid formation modulates the biological activity of a bacterial protein. J. Biol. Chem. 280:26880-26885. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Braun, V., S. I. Patzer, and K. Hantke. 2002. Ton-dependent colicins and microcins: modular design and evolution. Biochimie 84:365-380. - PubMed
-
- Brundage, L., J. P. Hendrick, E. Schiebel, A. J. Driessen, and W. Wickner. 1990. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell 62:649-657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

