Adaptive gene expression in Bacillus subtilis strains deleted for tetL
- PMID: 17015648
- PMCID: PMC1636236
- DOI: 10.1128/JB.00885-06
Adaptive gene expression in Bacillus subtilis strains deleted for tetL
Abstract
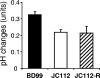
The growth properties of a new panel of Bacillus subtilis tetL deletion strains and of a derivative set of strains in which tetL is restored to the chromosome support earlier indications that deletion of tetL results in a range of phenotypes that are unrelated to tetracycline resistance. These phenotypes were not reversed by restoration of a tetL gene to its native locus and were hypothesized to result from secondary mutations that arise when multifunctional tetL is deleted. Such genetic changes would temper the alkali sensitivity and Na(+) sensitivity that accompany loss of the monovalent cation/proton activity of TetL. Microarray comparisons of the transcriptomes of wild-type B. subtilis, a tetL deletion strain, and its tetL-restored derivative showed that 37 up-regulated genes and 13 down-regulated genes in the deletion strain did not change back to wild-type expression patterns after tetL was returned to the chromosome. Up-regulation of the citM gene, which encodes a divalent metal ion-coupled citrate transporter, was shown to account for the Co(2+)-sensitive phenotype of tetL mutants. The changes in expression of citM and genes encoding other ion-coupled solute transporters appear to be adaptive to loss of TetL functions in alkali and Na(+) tolerance, because they reduce Na(+)-coupled solute uptake and enhance solute uptake that is coupled to H(+) entry.
Figures


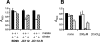
Similar articles
-
Two types of Bacillus subtilis tetA(L) deletion strains reveal the physiological importance of TetA(L) in K(+) acquisition as well as in Na(+), alkali, and tetracycline resistance.J Bacteriol. 2000 Apr;182(8):2088-95. doi: 10.1128/JB.182.8.2088-2095.2000. J Bacteriol. 2000. PMID: 10735849 Free PMC article.
-
Functions of tetracycline efflux proteins that do not involve tetracycline.J Mol Microbiol Biotechnol. 2001 Apr;3(2):237-46. J Mol Microbiol Biotechnol. 2001. PMID: 11321579 Review.
-
Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain.J Bacteriol. 1996 May;178(10):2853-60. doi: 10.1128/jb.178.10.2853-2860.1996. J Bacteriol. 1996. PMID: 8631673 Free PMC article.
-
TetL tetracycline efflux protein from Bacillus subtilis is a dimer in the membrane and in detergent solution.Biochemistry. 2003 Dec 2;42(47):13969-76. doi: 10.1021/bi035173q. Biochemistry. 2003. PMID: 14636065 Free PMC article.
-
The role of monovalent cation/proton antiporters in Na(+)-resistance and pH homeostasis in Bacillus: an alkaliphile versus a neutralophile.J Exp Biol. 1994 Nov;196:457-70. doi: 10.1242/jeb.196.1.457. J Exp Biol. 1994. PMID: 7823040 Review.
Cited by
-
Cytoplasmic acidification and the benzoate transcriptome in Bacillus subtilis.PLoS One. 2009 Dec 14;4(12):e8255. doi: 10.1371/journal.pone.0008255. PLoS One. 2009. PMID: 20011599 Free PMC article.
-
Acid and base stress and transcriptomic responses in Bacillus subtilis.Appl Environ Microbiol. 2009 Feb;75(4):981-90. doi: 10.1128/AEM.01652-08. Epub 2008 Dec 29. Appl Environ Microbiol. 2009. PMID: 19114526 Free PMC article.
-
Tetracycline resistance-encoding plasmid from Bacillus sp. strain #24, isolated from the marine sponge Haliclona simulans.Appl Environ Microbiol. 2011 Jan;77(1):327-9. doi: 10.1128/AEM.01239-10. Epub 2010 Nov 5. Appl Environ Microbiol. 2011. PMID: 21057017 Free PMC article.
-
In-Depth Genomic and Phenotypic Characterization of the Antarctic Psychrotolerant Strain Pseudomonas sp. MPC6 Reveals Unique Metabolic Features, Plasticity, and Biotechnological Potential.Front Microbiol. 2019 May 24;10:1154. doi: 10.3389/fmicb.2019.01154. eCollection 2019. Front Microbiol. 2019. PMID: 31178851 Free PMC article.
-
Detection of a Novel, and Likely Ancestral, Tn916-Like Element from a Human Saliva Metagenomic Library.Genes (Basel). 2020 May 14;11(5):548. doi: 10.3390/genes11050548. Genes (Basel). 2020. PMID: 32422869 Free PMC article.
References
-
- Bordi, C., L. Theraulaz, V. Mejean, and C. Jourlin-Castelli. 2003. Anticipating an alkaline stress through the Tor phosphorelay system in Escherichia coli. Mol. Microbiol. 48:211-223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

