Ultrafast infrared spectroscopy reveals a key step for successful entry into the photocycle for photoactive yellow protein
- PMID: 17015839
- PMCID: PMC1940041
- DOI: 10.1073/pnas.0603476103
Ultrafast infrared spectroscopy reveals a key step for successful entry into the photocycle for photoactive yellow protein
Abstract
Photoactive proteins such as PYP (photoactive yellow protein) are generally accepted as model systems for studying protein signal state formation. PYP is a blue-light sensor from the bacterium Halorhodospira halophila. The formation of PYP's signaling state is initiated by trans-cis isomerization of the p-coumaric acid chromophore upon the absorption of light. The quantum yield of signaling state formation is approximately 0.3. Using femtosecond visible pump/mid-IR probe spectroscopy, we investigated the structure of the very short-lived ground state intermediate (GSI) that results from an unsuccessful attempt to enter the photocycle. This intermediate and the first stable GSI on pathway into the photocycle, I0, both have a mid-IR difference spectrum that is characteristic of a cis isomer, but only the I0 intermediate has a chromophore with a broken hydrogen bond with the backbone N atom of Cys-69. We suggest, therefore, that breaking this hydrogen bond is decisive for a successful entry into the photocycle. The chromophore also engages in a hydrogen-bonding network by means of its phenolate group with residues Tyr-42 and Glu-46. We have investigated the role of this hydrogen bond by exchanging the H bond-donating residue Glu-46 with the weaker H bond-donating glutamine (i.e., Gln-46). We have observed that this mutant exhibits virtually identical kinetics and product yields as WT PYP, even though during the I0-to-I1 transition, on the 800-ps time scale, the hydrogen bond of the chromophore with Gln-46 is broken, whereas this hydrogen bond remains intact with Glu-46.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
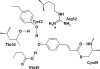
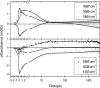
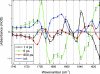

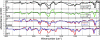
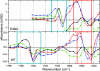
 O (blue box) and chromophore C
O (blue box) and chromophore C O (red box) regions.
O (red box) regions.References
-
- Hellingwerf KJ, Hendriks J, Gensch T. J Phys Chem A. 2003;107:1082–1094.
-
- Cusanovich MA, Meyer TE. Biochemistry. 2003;42:4759–4770. - PubMed
-
- Larsen DS, van Grondelle R. Chem Phys Chem. 2005;6:828–837. - PubMed
-
- Chosrowjan H, Mataga N, Nakashima N, Yasushi I, Tokunaga F. Chem Phys Lett. 1997;270:267–272.
-
- Mataga N, Chosrowjan H, Shibata Y, Imamoto Y, Tokunaga F. J Phys Chem B. 2000;104:5191–5199.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

