Molecular gymnastics at the herpesvirus surface
- PMID: 17016458
- PMCID: PMC1618366
- DOI: 10.1038/sj.embor.7400807
Molecular gymnastics at the herpesvirus surface
Abstract
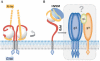
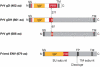
This review analyses recent structural results that provide clues about a possible molecular mechanism for the transmission of a fusogenic signal among the envelope glycoproteins of the herpes simplex virus on receptor binding by glycoprotein gD. This signal triggers the membrane-fusion machinery of the virus--contained in glycoproteins gB, gH and gL--to induce the merging of viral and cellular membranes, and to allow virus entry into target cells. This activating process parallels that of gamma-retroviruses, in which receptor binding by the amino-terminal domain of the envelope protein activates the fusogenic potential of the virion in a similar way, despite the different organization of the envelope complexes of these two types of viruses. Therefore, the new structural results on the interaction of gD with its receptors might also provide insights into the mechanism of fusogenic signal transmission in gamma-retroviruses. Furthermore, the fusion activation parallels with retroviruses, together with the recently reported structural homology of gB with the rhabdovirus envelope glycoprotein indicate that the complex entry apparatus of herpesviruses appears to be functionally related to that of simpler enveloped viruses.
Figures
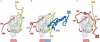

References
-
- Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC (2001) Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8: 169–179 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

