Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide?
- PMID: 17016507
- PMCID: PMC2014646
- DOI: 10.1038/sj.bjp.0706906
Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide?
Abstract
Background and purpose: The aim of these experiments was to evaluate the significance of the chemical reaction between hydrogen sulphide (H2S) and nitric oxide (NO) for the control of vascular tone.
Experimental approach: The effect of sodium hydrosulphide (NaHS; H2S donor) and a range of NO donors, such as sodium nitroprusside (SNP), either alone or together, was determined using phenylephrine (PE)-precontracted rat aortic rings and on the blood pressure of anaesthetised rats.
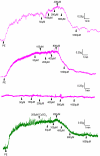
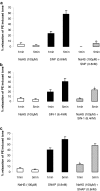
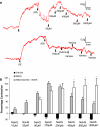
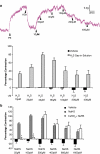
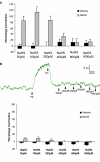
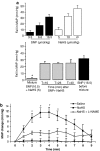
Key results: Mixing NaHS with NO donors inhibited the vasorelaxant effect of NO both in vitro and in vivo. Low concentrations of NaHS or H2S gas in solution reversed the relaxant effect of acetylcholine (ACh, 400 nM) and histamine (100 microM) but not isoprenaline (400 nM). The effect of NaHS on the ACh response was antagonized by CuSO(4) (200 nM) but was unaffected by glibenclamide (10 microM). In contrast, high concentrations of NaHS (200-1600 microM) relaxed aortic rings directly, an effect reduced by glibenclamide but unaffected by CuSO4. Intravenous infusion of a low concentration of NaHS (10 micromol kg(-1) min(-1)) into the anaesthetized rat significantly increased mean arterial blood pressure. L-NAME (25 mg kg(-1), i.v.) pretreatment reduced this effect.
Conclusions and implications: These results suggest that H2S and NO react together to form a molecule (possibly a nitrosothiol) which exhibits little or no vasorelaxant activity either in vitro or in vivo. We propose that a crucial, and hitherto unappreciated, role of H2S in the vascular system is the regulation of the availability of NO.
Figures
Comment in
-
What is the significance of vascular hydrogen sulphide (H2S)?Br J Pharmacol. 2006 Nov;149(6):609-10. doi: 10.1038/sj.bjp.0706907. Epub 2006 Oct 3. Br J Pharmacol. 2006. PMID: 17016506 Free PMC article.
References
-
- Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol. 2004;286:R678–R685. - PubMed
-
- Golpon HA, Puchner A, Barth P, Welte T, Wichert P, Feddersen CO. Nitric oxide-dependent vasorelaxation and endothelial cell damage caused by mercury chloride. Toxicology. 2003;192:179–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

