Deiodinases: implications of the local control of thyroid hormone action
- PMID: 17016550
- PMCID: PMC1578599
- DOI: 10.1172/JCI29812
Deiodinases: implications of the local control of thyroid hormone action
Abstract
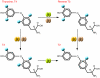
The deiodinases activate or inactivate thyroid hormone, and their importance in thyroid hormone homeostasis has become increasingly clear with the availability of deiodinase-deficient animals. At the same time, heightened interest in the field has been generated following the discovery that the type 2 deiodinase can be an important component in both the Hedgehog signaling pathway and the G protein-coupled bile acid receptor 1-mediated (GPBAR1-mediated) signaling cascade. The discovery of these new roles for the deiodinases indicates that tissue-specific deiodination plays a much broader role than once thought, extending into the realms of developmental biology and metabolism.
Figures

References
-
- Wu Y., Koenig R.J. Gene regulation by thyroid hormone. Trends Endocrinol. Metab. 2000;11:207–211. - PubMed
-
- Wondisford F.E. Thyroid hormone action: insight from transgenic mouse models. J. Investig. Med. 2003;51:215–220. - PubMed
-
- Yen P.M., et al. Thyroid hormone action at the cellular, genomic and target gene levels. Mol. Cell. Endocrinol. 2006;246:121–127. - PubMed
-
- St. Germain D.L., Galton V.A. The deiodinase family of selenoproteins. Thyroid. 1997;7:655–668. - PubMed
-
- Bianco A.C., Salvatore D., Gereben B., Berry M.J., Larsen P.R. Biochemistry, cellular and molecular biology and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002;23:38–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

