Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo
- PMID: 17016558
- PMCID: PMC1578628
- DOI: 10.1172/JCI29218
Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo
Abstract
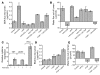
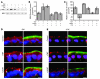
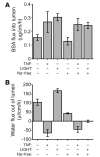
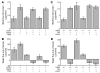
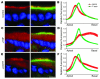
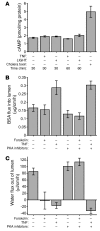
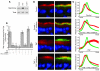
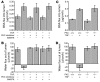
Acute T cell-mediated diarrhea is associated with increased mucosal expression of proinflammatory cytokines, including the TNF superfamily members TNF and LIGHT. While we have previously shown that epithelial barrier dysfunction induced by myosin light chain kinase (MLCK) is required for the development of diarrhea, MLCK inhibition does not completely restore water absorption. In contrast, although TNF-neutralizing antibodies completely restore water absorption after systemic T cell activation, barrier function is only partially corrected. This suggests that, while barrier dysfunction is critical, other processes must be involved in T cell-mediated diarrhea. To define these processes in vivo, we asked whether individual cytokines might regulate different events in T cell-mediated diarrhea. Both TNF and LIGHT caused MLCK-dependent barrier dysfunction. However, while TNF caused diarrhea, LIGHT enhanced intestinal water absorption. Moreover, TNF, but not LIGHT, inhibited Na+ absorption due to TNF-induced internalization of the brush border Na+/H+ exchanger NHE3. LIGHT did not cause NHE3 internalization. PKCalpha activation by TNF was responsible for NHE3 internalization, and pharmacological or genetic PKCalpha inhibition prevented NHE3 internalization, Na+ malabsorption, and diarrhea despite continued barrier dysfunction. These data demonstrate the necessity of coordinated Na+ malabsorption and barrier dysfunction in TNF-induced diarrhea and provide insight into mechanisms of intestinal water transport.
Figures
Comment in
-
T cell activation alters intestinal structure and function.J Clin Invest. 2006 Oct;116(10):2580-2. doi: 10.1172/JCI29985. J Clin Invest. 2006. PMID: 17016551 Free PMC article.
Similar articles
-
T cell activation alters intestinal structure and function.J Clin Invest. 2006 Oct;116(10):2580-2. doi: 10.1172/JCI29985. J Clin Invest. 2006. PMID: 17016551 Free PMC article.
-
IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction.Gastroenterology. 2006 Oct;131(4):1153-63. doi: 10.1053/j.gastro.2006.08.022. Epub 2006 Aug 22. Gastroenterology. 2006. PMID: 17030185 Free PMC article.
-
LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms.Gastroenterology. 2007 Jun;132(7):2383-94. doi: 10.1053/j.gastro.2007.02.052. Epub 2007 Feb 27. Gastroenterology. 2007. PMID: 17570213 Free PMC article.
-
Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application.Am J Pathol. 2006 Dec;169(6):1901-9. doi: 10.2353/ajpath.2006.060681. Am J Pathol. 2006. PMID: 17148655 Free PMC article. Review.
-
Beyond Ussing's chambers: contemporary thoughts on integration of transepithelial transport.Am J Physiol Cell Physiol. 2016 Mar 15;310(6):C423-31. doi: 10.1152/ajpcell.00348.2015. Epub 2015 Dec 23. Am J Physiol Cell Physiol. 2016. PMID: 26702131 Free PMC article. Review.
Cited by
-
Duodenal acidification induces gastric relaxation and alters epithelial barrier function by a mast cell independent mechanism.Sci Rep. 2020 Oct 15;10(1):17448. doi: 10.1038/s41598-020-74491-1. Sci Rep. 2020. PMID: 33060783 Free PMC article. Clinical Trial.
-
Electrophysiologic Analysis of Tight Junction Size and Charge Selectivity.Curr Protoc. 2021 Jun;1(6):e143. doi: 10.1002/cpz1.143. Curr Protoc. 2021. PMID: 34106526 Free PMC article.
-
Intestinal Epithelial Digestive, Transport, and Barrier Protein Expression Is Increased in Environmental Enteric Dysfunction.Lab Invest. 2023 Apr;103(4):100036. doi: 10.1016/j.labinv.2022.100036. Epub 2023 Jan 10. Lab Invest. 2023. PMID: 36870290 Free PMC article.
-
Systemic Inflammatory Protein Profiles Distinguish Irritable Bowel Syndrome (IBS) and Ulcerative Colitis, Irrespective of Inflammation or IBS-Like Symptoms.Inflamm Bowel Dis. 2020 May 12;26(6):874-884. doi: 10.1093/ibd/izz322. Inflamm Bowel Dis. 2020. PMID: 31901089 Free PMC article.
-
Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux.Mol Biol Cell. 2013 Oct;24(19):3056-68. doi: 10.1091/mbc.E12-09-0688. Epub 2013 Aug 7. Mol Biol Cell. 2013. PMID: 23924897 Free PMC article.
References
-
- Hawker P.C., McKay J.S., Turnberg L.A. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980;79:508–511. - PubMed
-
- Ferran C., et al. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur. J. Immunol. 1990;20:509–515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

