Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect
- PMID: 17016561
- PMCID: PMC1578623
- DOI: 10.1172/JCI28310
Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect
Abstract
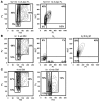
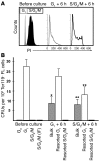
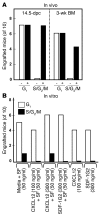
The regulation of HSC proliferation and engraftment of the BM is an important but poorly understood process, particularly during ontogeny. Here we show that in mice, all HSCs are cycling until 3 weeks after birth. Then, within 1 week, most became quiescent. Prior to 4 weeks of age, the proliferating HSCs with long-term multilineage repopulating activity displayed an engraftment defect when transiting S/G2/M. During these cell cycle phases, their expression of CXC chemokine ligand 12 (CXCL12; also referred to as stromal cell-derived factor 1 [SDF-1]) transiently increased. The defective engrafting activity of HSCs in S/G2/M was reversed when cells were allowed to progress into G1 prior to injection or when the hosts (but not the cells) were pretreated with a CXCL12 antagonist. Interestingly, the enhancing effect of CXCL12 antagonist pretreatment was exclusive to transplants of long-term multilineage repopulating HSCs in S/G2/M. These results demonstrate what we believe to be a new HSC regulatory checkpoint during development. They also suggest an ability of HSCs to express CXCL12 in a fashion that changes with cell cycle progression and is associated with a defective engraftment that can be overcome by in vivo administration of a CXCL12 antagonist.
Figures




Comment in
-
Children are not little adults: just ask their hematopoietic stem cells.J Clin Invest. 2006 Oct;116(10):2593-6. doi: 10.1172/JCI30083. J Clin Invest. 2006. PMID: 17016556 Free PMC article.
References
-
- Leemhuis T., et al. Isolation of primitive human bone marrow hematopoietic progenitor cells using Hoechst 33342 and Rhodamine 123. Exp. Hematol. 1996;24:1215–1224. - PubMed
-
- Osawa M., Hanada K., Hamada H., Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

