VSIG4, a B7 family-related protein, is a negative regulator of T cell activation
- PMID: 17016562
- PMCID: PMC1578631
- DOI: 10.1172/JCI25673
VSIG4, a B7 family-related protein, is a negative regulator of T cell activation
Abstract
T cell activation by APCs is positively and negatively regulated by members of the B7 family. We have identified a previously unknown function for B7 family-related protein V-set and Ig domain-containing 4 (VSIG4). In vitro experiments using VSIG4-Ig fusion molecules showed that VSIG4 is a strong negative regulator of murine and human T cell proliferation and IL-2 production. Administration to mice of soluble VSIG4-Ig fusion molecules reduced the induction of T cell responses in vivo and inhibited the production of Th cell-dependent IgG responses. Unlike that of B7 family members, surface expression of VSIG4 was restricted to resting tissue macrophages and absent upon activation by LPS or in autoimmune inflammatory foci. The specific expression of VSIG4 on resting macrophages in tissue suggests that this inhibitory ligand may be important for the maintenance of T cell unresponsiveness in healthy tissues.
Figures




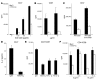

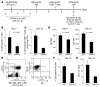
Comment in
-
To be or not to be B7.J Clin Invest. 2006 Oct;116(10):2590-3. doi: 10.1172/JCI30103. J Clin Invest. 2006. PMID: 17016555 Free PMC article.
References
-
- Langman R.E., Cohn M. Two signal models of lymphocyte activation? Immunol. Today. 1993;14:235–237. - PubMed
-
- Lenschow D.J., Walunas T.L., Bluestone J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. - PubMed
-
- Chambers C.A., Allison J.P. Costimulatory regulation of T cell function. Curr. Opin. Cell Biol. 1999;11:203–210. - PubMed
-
- Schwartz R.H. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. - PubMed
-
- Sharpe A.H., Freeman G.J. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002;2:116–126. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

