Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric
- PMID: 17018142
- PMCID: PMC1621073
- DOI: 10.1186/1471-2202-7-66
Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric
Abstract
Background: Glaucoma is a chronic neurodegenerative disease of the retina, characterized by the degeneration of axons in the optic nerve and retinal ganglion cell apoptosis. DBA/2J inbred mice develop chronic hereditary glaucoma and are an important model system to study the molecular mechanisms underlying this disease and novel therapeutic interventions designed to attenuate the loss of retinal ganglion cells. Although the genetics of this disease in these mice are well characterized, the etiology of its progression, particularly with respect to retinal degeneration, is not. We have used two separate labeling techniques, post-mortem DiI labeling of axons and ganglion cell-specific expression of the betaGeo reporter gene, to evaluate the time course of optic nerve degeneration and ganglion cell loss, respectively, in aging mice.
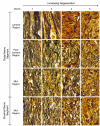
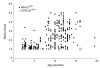
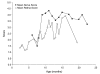
Results: Optic nerve degeneration, characterized by axon loss and gliosis is first apparent in mice between 8 and 9 months of age. Degeneration appears to follow a retrograde course with axons dying from their proximal ends toward the globe. Although nerve damage is typically bilateral, the progression of disease is asymmetric between the eyes of individual mice. Some nerves also exhibit focal preservation of tracts of axons generally in the nasal peripheral region. Ganglion cell loss, as a function of the loss of betaGeo expression, is evident in some mice between 8 and 10 months of age and is prevalent in the majority of mice older than 10.5 months. Most eyes display a uniform loss of ganglion cells throughout the retina, but many younger mice exhibit focal loss of cells in sectors extending from the optic nerve head to the retinal periphery. Similar to what we observe in the optic nerves, ganglion cell loss is often asymmetric between the eyes of the same animal.
Conclusion: A comparison of the data collected from the two cohorts of mice used for this study suggests that the initial site of damage in this disease is to the axons in the optic nerve, followed by the subsequent death of the ganglion cell soma.
Figures








References
-
- John SWM, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. - PubMed
-
- Mo JS, Anderson MG, Gregory M, Smith RS, Savinova OV, Sereze DV, Ksander BR, Streilein JW, John SWM. By altering ocular immune privilege, bone marrow-derived cells pathogenetically contribute to DBA/2J pigmentary glaucoma. J Exp Med. 2003;197:1335–1344. doi: 10.1084/jem.20022041. - DOI - PMC - PubMed
-
- Libby RT, Anderson MG, Pang IH, Robinson Z, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SWM. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

