Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression
- PMID: 17018296
- PMCID: PMC2923825
- DOI: 10.1016/j.molcel.2006.07.033
Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression
Abstract
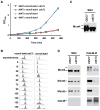
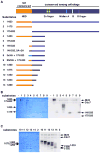
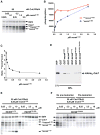
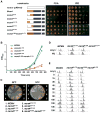
Origins of DNA replication are licensed in G1 by recruiting the minichromosome maintenance (MCM) proteins to form a prereplicative complex (pre-RC). Prior to initiation of DNA synthesis from each origin, a preinitiation complex (pre-IC) containing Cdc45 and other proteins is formed. We report that Cdc7-Dbf4 protein kinase (DDK) promotes assembly of a stable Cdc45-MCM complex exclusively on chromatin in S phase. In this complex, Mcm4 is hyperphosphorylated. Studies in vitro using purified DDK and Mcm4 demonstrate that hyperphosphorylation occurs at the Mcm4 N terminus. However, the DDK substrate specificity is conferred by an adjacent DDK-docking domain (DDD), sufficient for facilitating efficient phosphorylation of artificial phosphoacceptors in cis. Genetic evidence suggests that phosphorylation of Mcm4 by DDK is important for timely S phase progression and for cell viability upon overproduction of Cdc45. We suggest that DDK docks on and phosphorylates MCM proteins at licensed origins to promote proper assembly of pre-IC.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

