Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells
- PMID: 17018840
- PMCID: PMC2672957
- DOI: 10.1152/ajprenal.00302.2006
Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells
Abstract
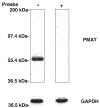
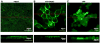
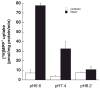
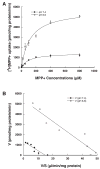
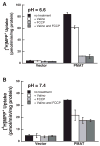
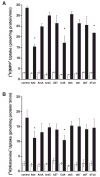
Plasma membrane monoamine transporter (PMAT) is a novel membrane transporter recently cloned and characterized in our laboratory. We previously demonstrated that PMAT functions as a polyspecific organic cation transporter and efficiently transports many organic cations such as monoamine neurotransmitters and 1-methyl-4-phenylpyridinium (MPP(+)). In this study, we explored the role of PMAT in the renal handling of organic cations. Using a polyclonal antibody generated toward the NH(2)-terminal 66 amino acid residues of human PMAT, we showed that the PMAT protein (approximately 55 kDa) is expressed in the human kidney and is primarily targeted to the apical membranes when expressed in polarized Madin-Darby canine kidney (MDCK) cells. Using MDCK cells stably expressing human PMAT, we showed that PMAT-mediated MPP(+) uptake is strongly dependent on extracellular pH. Lowering extracellular pH from 7.4 to 6.6 greatly stimulated PMAT-mediated MPP(+) uptake, whereas elevating extracellular pH to 8.2 abolished transporter activity. Kinetic analysis revealed that the apparent V(max) at pH 6.6 is about fourfold higher than that at pH 7.4, whereas the apparent K(m) values were not statistically different at these two conditions. Under acidic conditions (pH 6.6), the proton ionophore, carbonyl cyanide p-trifluormethoxyphenylhydrazone, drastically reduced PMAT-mediated MPP(+) uptake, suggesting that the stimulatory effect of proton may be due to transporter coupling with a proton gradient. Taken together, our data suggest that PMAT is expressed on the apical membranes of renal epithelial cells and may use luminal proton gradient to drive organic cation reabsorption in the kidney.
Figures

Similar articles
-
Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine.Drug Metab Dispos. 2007 Oct;35(10):1956-62. doi: 10.1124/dmd.107.015495. Epub 2007 Jun 28. Drug Metab Dispos. 2007. PMID: 17600084 Free PMC article.
-
Adenosine transport by plasma membrane monoamine transporter: reinvestigation and comparison with organic cations.Drug Metab Dispos. 2010 Oct;38(10):1798-805. doi: 10.1124/dmd.110.032987. Epub 2010 Jun 30. Drug Metab Dispos. 2010. PMID: 20592246 Free PMC article.
-
Interaction of organic cations with a newly identified plasma membrane monoamine transporter.Mol Pharmacol. 2005 Nov;68(5):1397-407. doi: 10.1124/mol.105.016832. Epub 2005 Aug 11. Mol Pharmacol. 2005. PMID: 16099839
-
Histamine Clearance Through Polyspecific Transporters in the Brain.Handb Exp Pharmacol. 2017;241:173-187. doi: 10.1007/164_2016_13. Handb Exp Pharmacol. 2017. PMID: 27679412 Review.
-
The plasma membrane monoamine transporter (PMAT): Structure, function, and role in organic cation disposition.Clin Pharmacol Ther. 2016 Nov;100(5):489-499. doi: 10.1002/cpt.442. Epub 2016 Sep 19. Clin Pharmacol Ther. 2016. PMID: 27506881 Free PMC article. Review.
Cited by
-
Organic cation transporters in psychiatric and substance use disorders.Pharmacol Ther. 2024 Jan;253:108574. doi: 10.1016/j.pharmthera.2023.108574. Epub 2023 Dec 9. Pharmacol Ther. 2024. PMID: 38072333 Free PMC article. Review.
-
Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene.J Biol Chem. 2013 Feb 1;288(5):3535-44. doi: 10.1074/jbc.M112.436972. Epub 2012 Dec 19. J Biol Chem. 2013. PMID: 23255610 Free PMC article.
-
The Mechanism of Action of Biguanides: New Answers to a Complex Question.Cancers (Basel). 2022 Jun 30;14(13):3220. doi: 10.3390/cancers14133220. Cancers (Basel). 2022. PMID: 35804992 Free PMC article. Review.
-
The Effect of Famotidine, a MATE1-Selective Inhibitor, on the Pharmacokinetics and Pharmacodynamics of Metformin.Clin Pharmacokinet. 2016 Jun;55(6):711-21. doi: 10.1007/s40262-015-0346-3. Clin Pharmacokinet. 2016. PMID: 26597253 Free PMC article. Clinical Trial.
-
Solute Carrier Nucleoside Transporters in Hematopoiesis and Hematological Drug Toxicities: A Perspective.Cancers (Basel). 2022 Jun 25;14(13):3113. doi: 10.3390/cancers14133113. Cancers (Basel). 2022. PMID: 35804885 Free PMC article. Review.
References
-
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflügers Arch. 2004;447:735–743. - PubMed
-
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, Ritzel MW, Cass CE, Young JD. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280:15880–15887. - PubMed
-
- Chen R, Pan BF, Sakurai M, Nelson JA. A nucleoside-sensitive organic cation transporter in opossum kidney cells. Am J Physiol Renal Physiol. 1999;276:F323–F328. - PubMed
-
- Engel K, Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68:1397–1407. - PubMed
-
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

