Ischemia leads to delayed union during fracture healing: a mouse model
- PMID: 17019699
- PMCID: PMC2848995
- DOI: 10.1002/jor.20264
Ischemia leads to delayed union during fracture healing: a mouse model
Abstract
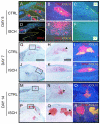
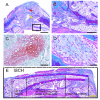
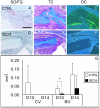
Vascular damage accompanying skeletal injury leads to an ischemic environment, and in clinical settings the extent of vascular damage is directly correlated with failure of skeletal repair. However, the exact mechanism(s) underlying ischemia-related defects in bone healing are not well understood. To better understand the mechanism and to facilitate development of novel interventions to treat ischemic fractures, a mouse model of long bone fracture healing in an ischemic environment was created. Ischemia was induced by femoral artery resection prior to tibia fracture. Fractures were left unstabilized or were stabilized with custom-designed external fixators. Animals with intact femoral vessels served as controls. Tissues from non-stabilized fractures were analyzed at various times from 3 to 28 days after injury (n = 5/time point). Femoral artery resection severely impaired blood supply to the fractured limbs, and perfusion to the fracture sites did not recover until 14 days post-injury. Ischemia significantly decreased the callus size (p < 0.05), and decreased bone (p < 0.05) and cartilage (p < 0.05) matrix production during healing of non-stabilized fracture. The decreased formation of skeletal tissues in ischemic limbs was accompanied by decreased cell proliferation and increased apoptosis at early time points, and increased fibrous and fatty tissues adjacent to the fracture site during the third and fourth week after injury. These alterations led to a delayed-union. Complete fracture healing was not achieved in the majority (day 21 = 4/5; day 28 = 5/5) of ischemic animals, while all control mice (n = 5/5) had evidence of bony bridging by day 21. The ratio of cartilage to bone was similar in ischemic and control limbs at days 7 and 10 in non-stabilized fractures. In stabilized fractures, which healed through direct bone formation in the nonischemic controls, ischemia decreased the amount of bone formation at days 10 and 14 (n = 5/time point) but did not induce cartilage formation. These data reveal that an ischemic insult in the hind limb prior to fracture leads to a delayed union or a nonunion, but does not favor formation of cartilage over bone. This model will be useful for testing novel therapeutic regimens to stimulate fracture healing.
(c) 2006 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures



References
-
- Bassett CA, Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature. 1961;190:460–1. - PubMed
-
- Choi P, Ogilvie C, Thompson Z, et al. Cellular and molecular characterization of a murine non-union model. J Orthop Res. 2004;22:1100–7. - PubMed
-
- Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemp Orthop. 1995;30:489–93. - PubMed
-
- Domm C, Schunke M, Christesen K, et al. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteo Cart. 2002;10:13–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

