Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex
- PMID: 17021176
- PMCID: PMC6674642
- DOI: 10.1523/JNEUROSCI.3028-06.2006
Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex
Abstract
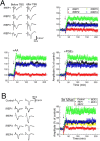
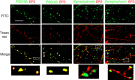
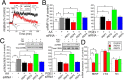
Although prostaglandin E2 (PGE2) has a broad spectrum of biological activities that have been confirmed by previous studies, the roles of PGE2 in synaptic plasticity such as long-term potentiation (LTP) in the CNS have yet to be characterized in detail. The present results of electrophysiological and biochemical studies indicated that PGE2 is actually produced in acute visual cortex slices in response to theta-burst stimulation (TBS) and is involved postsynaptically in TBS-induced LTP. RNA interference (RNAi) for PGE2 receptor subtypes EP2 and EP3, which are known to upregulate and downregulate the level of cAMP, respectively, induced significant decreases and increases of LTP, respectively. Moreover, analysis of the localization of receptor subtypes at the membrane surface or cytosol showed that stimuli such as TBS regulate the trafficking of EP2 and EP3 between the membrane and cytosol of the postsynapses, rising up to and leaving the membrane, respectively, resulting in increased and decreased expression of EP2 and EP3 at the membrane, respectively. Increased activation of EP2 and decreased activation of EP3 by PGE2 synergistically induce an increase in cAMP level, which may induce LTP. This causes activation of CREB (cAMP response element-binding protein) in the postsynaptic cells, which may be involved in the maintenance of LTP. These observations indicate that in TBS-induced LTP of the visual cortex, PGE2 is released from the postsynaptic cells and then activates PGE2 receptors at the postsynaptic membranes, which is regulated by trafficking of the differential PGE2 receptor subtypes in an activity-dependent bidirectional manner.
Figures






References
-
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. - PubMed
-
- Akaneya A, Jiang B, Tsumoto T. RNAi-induced gene silencing by local electroporation in targeting brain region. J Neurophysiol. 2005;93:594–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
