Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis
- PMID: 17021177
- PMCID: PMC6674636
- DOI: 10.1523/JNEUROSCI.2250-06.2006
Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis
Abstract
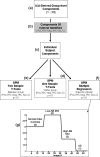
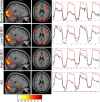
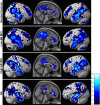
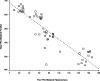
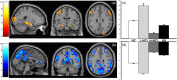
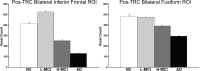
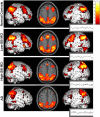
Memory function is likely subserved by multiple distributed neural networks, which are disrupted by the pathophysiological process of Alzheimer's disease (AD). In this study, we used multivariate analytic techniques to investigate memory-related functional magnetic resonance imaging (fMRI) activity in 52 individuals across the continuum of normal aging, mild cognitive impairment (MCI), and mild AD. Independent component analyses revealed specific memory-related networks that activated or deactivated during an associative memory paradigm. Across all subjects, hippocampal activation and parietal deactivation demonstrated a strong reciprocal relationship. Furthermore, we found evidence of a nonlinear trajectory of fMRI activation across the continuum of impairment. Less impaired MCI subjects showed paradoxical hyperactivation in the hippocampus compared with controls, whereas more impaired MCI subjects demonstrated significant hypoactivation, similar to the levels observed in the mild AD subjects. We found a remarkably parallel curve in the pattern of memory-related deactivation in medial and lateral parietal regions with greater deactivation in less-impaired MCI and loss of deactivation in more impaired MCI and mild AD subjects. Interestingly, the failure of deactivation in these regions was also associated with increased positive activity in a neocortical attentional network in MCI and AD. Our findings suggest that loss of functional integrity of the hippocampal-based memory systems is directly related to alterations of neural activity in parietal regions seen over the course of MCI and AD. These data may also provide functional evidence of the interaction between neocortical and medial temporal lobe pathology in early AD.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
